Abstract
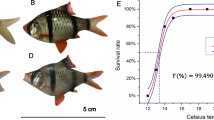
The northern quahog (Mercenaria mercenaria) supports lucrative aquaculture industries in the USA. In the southeastern USA, aquacultured M. mercenaria faces increasing risks of summer die-offs from prolonged heat waves. We used a comparative transcriptomic approach to investigate the molecular responses of M. mercenaria and its southern congener, Mercenaria campechiensis, to controlled incremental heat stress over a 4-week period. Mercenaria were exposed to temperatures from 24 to 34 °C with 2.5 °C/week, after which, gill transcriptomes were de novo assembled and annotated. During the 4 weeks of chronic heat exposure, both species had the same survival rate (96%); M. mercenaria experienced body weight gain/loss depending on the originated hatcheries while M. campechiensis experienced an average net weight loss. The upregulated genes in both species included those in chaperone-mediated protein folding and regulation of cell death pathways, while the downregulated genes in both species involved in mRNA processing and splicing pathways. Compared to M. mercenaria, M. campechiensis appears to be more sensitive to prolonged heat stress as indicated by upregulating significantly more genes in coping with oxidative stress and in the protein degradation pathways, while downregulating some inhibitors of apoptosis. We discussed this finding within their ecological and evolutionary context. Our findings highlighted the potential vulnerability of the two quahogs, especially the southern quahog, to continued ocean warming.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw sequence data for this project have been uploaded to National Center for Biotechnology Information (NCBI) with a BioProject ID #PRJNA743680.
References
Abbott RT (1974) American seashells: The marine molluska of the Atlantic and Pacific coasts of North America, 2nd ed. Van Nostrand Reinhold Inc, New York, NY
Abele D, Heise K, Pörtner HO, Puntarulo S (2002) Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol 205:1831–1841
Alexa A, Rahnenführer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22:1600–1607
Andrews S (2015) FASTQC A Quality control tool for high throughput sequence data. In: Babraham Institute. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/Help/3AnalysisModules/
Arnold WS, Bert TM, Marelli DC et al (1996) Genotype-specific growth of hard clams (genus Mercenaria) in a hybrid zone: variation among habitats. Mar Biol 125:129–139
Arnold WS, Geiger SP, Stephenson SP (2009) Mercenaria mercenaria introductions into Florida, USA, waters: Duration, not size of introduction, influences genetic outcomes. Aquat Biol 5:49–62
Barcia R, Lopez-García JM, Ramos-Martínez JI (1997) The 28S fraction of rRNA in molluscs displays electrophoretic behaviour different from that of mammal cells. Biochem Mol Biol Int 42:1089–1092
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser. B Methodol 57:289–300
Bray NL, Pimentel H, Melsted P, Pachter L (2016) Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34:525–527
Bryant DM, Johnson K, DiTommaso T et al (2017) A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep 18:762–776
Chen M, Yang H, Delaporte M, Zhao S (2007) Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture 271:479–487
Chen N, Huang Z, Lu C et al (2019) Different transcriptomic responses to thermal stress in heat-tolerant and heat-sensitive pacific abalones indicated by cardiac performance. Front Physiol 10:1–14
Chew KK (2001) Introduction of the hard clam (Mercenaria mercenaria) to the Pacific coast of North America with notes on its introduction to Puerto Rico, England, and France. Chapter 16. In: Kraeuter JN, Castagna M (eds) Developments in aquaculture and fisheries science Volume 31, Biology of the hard clam. Elsevier, Amsterdam, The Netherlands, pp 701–709
Dabbaghizadeh A, Tanguay RM (2020) Structural and functional properties of proteins interacting with small heat shock proteins. Cell Stress Chaperones 25:629–637
DeBiasse MB, Kelly MW (2016) Plastic and evolved responses to global change: What can we learn from comparative transcriptomics? J Hered 107:71–81
Deutsch CA, Tewksbury JJ, Huey RB et al (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci 105:6668–6672
Dillon RT (1992) Minimal hybridization between populations of the hard clams, Mercenaria mercenaria and Mercenaria campechiensis, co-occurring in South Carolina. Bull Mar Sci 50:411–416
Dong Y, Miller LP, Sanders JG, Somero GN (2008) Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: Interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull 215:173–181
Duarte H, Tejedo M, Katzenberger M et al (2012) Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob Change Biol 18:412–421
Elderkin CL, Klerks PL (2005) Variation in: thermal tolerance among three Mississippi River populations of the zebra mussel, Dreissena Polymorpha. J Shellfish Res 24:221–226
El-Wazzan E, Scarpa J (2009) Comparative growth of triploid and diploid juvenile hard clams Mercenaria mercenaria notata under controlled laboratory conditions. Aquaculture 289:236–243
Evans TG (2015) Considerations for the use of transcriptomics in identifying the ‘genes that matter’ for environmental adaptation. J Exp Biol 218:1925–1935
FAO (2020) The state of world fisheries and aquaculture. Sustainability in action, Rome. https://doi.org/10.4060/ca9229en
Froehlich HE, Gentry RR, Halpern BS (2018) Global change in marine aquaculture production potential under climate change. Nature Ecology & Evolution 2:1745–1750
Gjedrem T, Rye M (2018) Selection response in fish and shellfish: A review. Rev Aquac 10:168–179
Gupte R, Liu Z, Kraus WL (2017) PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev 31:101–126
Gurevich A, Saveliev V, Vyahhi N, Tesler G (2013) QUAST: Quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075
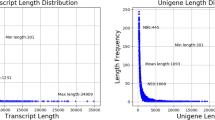
Haas BJ, Papanicolaou A, Yassour M et al (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Heekenda EJ, Austin JD, Zhang Z, Yang H (2020) Phenotypic and genetic identification of Mercenaria mercenaria, Mercenaria campechiensis, and their hybrids. J Shellfish Res 39:535–546
Hégaret H, Wikfors GH, Soudant P (2003) Flow cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation II. Haemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J Exp Mar Biol Ecol 293:249–265
Heise K, Puntarulo S, Nikinmaa M et al (2006) Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. J Exp Biol 209:353–363
Hollenbeck CM, Johnston IA (2018) Genomic tools and selective breeding in molluscs. Front Genet 9:1–15
Hu Z, Song H, Yang M, et al (2019) Transcriptome analysis of shell color-related genes in the hard clam Mercenaria mercenaria. Comp Biochem Physiol 31:100598. https://doi.org/10.1016/j.cbd.2019.100598
Ivanina AV, Dickinson GH, Matoo OB et al (2013) Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp Biochem Physiol 166:101–111
Jeffries KM, Hinch SG, Sierocinski T et al (2014) Transcriptomic responses to high water temperature in two species of Pacific salmon. Evol Appl 7:286–300
Ju S, Shaltiel G, Shamir A et al (2004) Human 1-D-myo-inositol-3-phosphate synthase is functional in yeast. J Biol Chem 279:21759–21765
Juárez OE, Lafarga-De la Cruz F, Leyva-Valencia I et al (2018) Transcriptomic and metabolic response to chronic and acute thermal exposure of juvenile geoduck clams Panopea globosa. Mar Genomics 42:1–13
Kim D, Paggi JM, Park C et al (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915
Kleizen B, Braakman I (2004) Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 16:343–349
Kültz D (2004) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Lang RP, Bayne CJ, Camara MD et al (2009) Transcriptome profiling of selectively bred Pacific oyster Crassostrea gigas families that differ in tolerance of heat shock. Mar Biotechnol 11:650–668
Li J, Zhang Y, Mao F et al (2017) Characterization and identification of differentially expressed genes involved in thermal adaptation of the Hong Kong oyster Crassostrea hongkongensis by digital gene expression profiling. Front Mar Sci 4:112
Lockwood BL, Sanders JG, Somero GN (2010) Transcriptomic responses to heat stress in invasive and native blue mussels (genus Mytilus): Molecular correlates of invasive success. J Exp Biol 213:3548–3558
Loosanoff VL (1954) New advances in the study of bivalve larvae. Am Sci 42:607–624
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
MacKenzie C, Taylor D, Arnold W (2001) A history of hard clamming. In: Kraeuter J, Castagna M (eds) Biology of the hard clam. Elsevier, Amsterdam, pp 651–674
Mangi SC, Lee J, Pinnegar JK et al (2018) The economic impacts of ocean acidification on shellfish fisheries and aquaculture in the United Kingdom. Environ Sci Policy 86:95–105
Menzel W (1977) Selection and hybridization in quahog clam Mercenaria spp. Proceedings of the Annual Meeting - World Mariculture Society 8:507–521
National Center for Environmental Information Coastal Water Temperature Guide. https://www.ncei.noaa.gov/access/coastal-water-temperature-guide/. Accessed 20 Aug 2021
Naylor RL, Hardy RW, Buschmann AH et al (2021) A 20-year retrospective review of global aquaculture. Nature 591:551–563
Pai AA, Luca F (2019) Environmental influences on RNA processing: Biochemical, molecular and genetic regulators of cellular response. Wiley Interdisciplinary Reviews RNA 10:e1503. https://doi.org/10.1002/wrna.1503
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62:1015–1026
Park H, Ahn IY, Hye EL (2007) Expression of heat shock protein 70 in the thermally stressed Antarctic clam Laternula elliptica. Cell Stress Chaperones 12:275–282
Priyam A, Woodcroft BJ, Rai V et al (2019) Sequenceserver: a modern graphical user interface for custom BLAST databases. Mol Biol Evol 36:2922–2924
R Core Team (2020) R: a language and environment for statistical computing, Vienna, Austria. https://www.R-project.org/
Rack JGM, Palazzo L, Ahel I (2020) (ADP-ribosyl) hydrolases: Structure, function, and biology. Genes Dev 34:263–284
Reverter M, Sarter S, Caruso D et al (2020) Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun 11:1870
Roberts RJ, Agius C, Saliba C et al (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J Fish Dis 33:789–801
Schnytzer Y, Simon-Blecher N, Li J et al (2018) Tidal and diel orchestration of behaviour and gene expression in an intertidal mollusc. Sci Rep 8:1–13
Shalgi R, Hurt JA, Lindquist S, Burge CB (2014) Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep 7:1362–1370
Simão FA, Waterhouse RM, Ioannidis P et al (2015) BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31:3210–3212
Song H, Guo X, Sun L et al (2021) The hard clam genome reveals massive expansion and diversification of inhibitors of apoptosis in Bivalvia. BMC Biol 19:15
Song J, McDowell JR (2021) Comparative transcriptomics of spotted seatrout (Cynoscion nebulosus) populations to cold and heat stress. Ecol Evol 11:1352–1367
Stewart-Sinclair PJ, Last KS, Payne BL, Wilding TA (2020) A global assessment of the vulnerability of shellfish aquaculture to climate change and ocean acidification. Ecol Evol 10:3518–3534
Sturmer LN, Scarpa J, White W, Baker S (2012) Improving hard clam production in Florida through culture of backcrossed hybrids (Mercenaria mercenaria, M. campechiensis). In: National Shellfisheries Association 104th Annual Meeting. p 351
Tan K, Zhang H, Zheng H (2020) Selective breeding of edible bivalves and its implication of global climate change. Rev Aquac 12:2559–2572
USDA (2019) 2018 Census of Aquaculture. https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Census_of_Aquaculture/index.php
Valenzuela-Castillo A, Sánchez-Paz A, Castro-Longoria R et al (2015) Seasonal changes in gene expression and polymorphism of hsp70 in cultivated oysters (Crassostrea gigas) at extreme temperatures. Mar Environ Res 110:25–32
Valluru R, Van den Ende W (2011) Myo-inositol and beyond - emerging networks under stress. Plant Sci 181:387–400
Wabnitz CCC, Lam VWY, Reygondeau G et al (2018) Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLoS ONE 13
Wang K, del Castillo C, Corre E et al (2016) Clam focal and systemic immune responses to QPX infection revealed by RNA-seq technology. BMC Genomics 17:1–22
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: A revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Weber K, Sturmer L, Hoover E, Baker S (2007) The role of water temperature in hard clam aquaculture, https://edis.ifas.ufl.edu/pdf/FA/FA15100.pdf
Yang H, Guo X (2018) Triploid hard clams Mercenaria mercenaria produced by inhibiting polar body I or polar body II. Aquac Res 49:449–461
Yost HJ, Lindquist S (1986) RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45:185–193
Zhang G, Li L, Meng J et al (2016) Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Biosci 4:357–381
Zhou C, Song H, Feng J et al (2021) RNA-Seq analysis and WGCNA reveal dynamic molecular responses to air exposure in the hard clam Mercenaria mercenaria. Genomics 113:2847–2859
Acknowledgements
The authors would like to thank Dr. Yanping Zhang for discussion with sample processing and RNA sequencing, and graduate student Jayme C Yee and research scientist Dr. Yuanzi Huo for help with quahog culture and feeding.
Funding
This study was supported by funds from National Sea Grant Aquaculture Program (NA18OAR4170344 and a sub-award 80794/3/1158304 for UF). Also, this study was partly supported by the Gulf States Marine Fisheries Commission (ACQ-210–039-2019-UFL) and the National Institute of Food and Agriculture, United States Department of Agriculture (Hatch project FLA-FOR-005385).
Author information
Authors and Affiliations
Contributions
JS: design of the work; acquisition, analysis, or interpretation of data; writing—original draft; writing—review and editing. JA: conception; interpretation of data; writing—review and editing; funding acquisition. HY: conception; design of the work; data curation; supervision; visualization; writing—original draft; writing—review and editing; funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, J., Austin, J.D. & Yang, H. Comparative Transcriptomics of the Northern Quahog Mercenaria mercenaria and Southern Quahog Mercenaria campechiensis in Response to Chronic Heat Stress. Mar Biotechnol 24, 276–292 (2022). https://doi.org/10.1007/s10126-022-10101-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10101-7