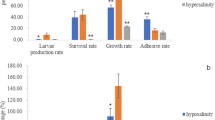
Abstract
Sessile inhabitants of marine intertidal environments commonly face heat stress, an important component of summer mortality syndrome in the Pacific oyster Crassostrea gigas. Marker-aided selection programs would be useful for developing oyster strains that resist summer mortality; however, there is currently a need to identify candidate genes associated with stress tolerance and to develop molecular markers associated with those genes. To identify candidate genes for further study, we used cDNA microarrays to test the hypothesis that oyster families that had high (>64%) or low (<29%) survival of heat shock (43°C, 1 h) differ in their transcriptional responses to stress. Based upon data generated by the microarray and by real-time quantitative PCR, we found that transcription after heat shock increased for genes putatively encoding heat shock proteins and genes for proteins that synthesize lipids, protect against bacterial infection, and regulate spawning, whereas transcription decreased for genes for proteins that mobilize lipids and detoxify reactive oxygen species. RNAs putatively identified as heat shock protein 27, collagen, peroxinectin, S-crystallin, and two genes with no match in Genbank had higher transcript concentrations in low-surviving families than in high-surviving families, whereas concentration of putative cystatin B mRNA was greater in high-surviving families. These ESTs should be studied further for use in marker-aided selection programs. Low survival of heat shock could result from a complex interaction of cell damage, opportunistic infection, and metabolic exhaustion.





Similar content being viewed by others
References
Abele D, Heise K, Pörtner HO, Puntarulo S (2002) Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol 205:1831–1841
Alexander WS (2002) Suppressors of cytokine signaling (SOCS) in the immune system. Nat Rev Immunol 6:410–416
Arnaud C, Joyeux M, Garrel C, Godin-Ribout D, Demenge P, Ribuot C (2002) Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Brit J Pharm 135:1776–1782
Benjamini Y, Hochberg Y (1995) Controling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Bengoechea-Alsono M, Ericcson J (2007) SREBP in signal transduction: cholesterol metabolism and beyond. Curr Op Cell Biol 19:215–222
Berthelin C, Kellner K, Mathieu M (2000) Storage metabolism in the Pacific oyster (Crassostrea gigas) in relationship to summer mortalities and reproductive cycle (West Coast of France). Comp Bio Phys B 125:359–369
Blanchette B, Feng X, Singh BR (2007) Marine glutathione S-transferases. Mar Biotech 9:513–542
Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D (2003) Molecular identification and transcription of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chap 8:76–85
Boutet I, Tanguy A, Moraga D (2004) Response of the Pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 329:147–157
Bruey J, Ducasse C, Bonniaud P, Ravagnan L, Susin S, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652
Bruskov V, Malakhova L, Masalimov Z, Chernikov A (2002) Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nuc Ac Res 30:1354–1363
Chen M, Yang H, Delaporte M, Zhao S (2007) Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture 271:479–487
Cheney D, MacDonald B, Elston R (2000) Summer mortality of Pacific oysters, Crassostrea gigas (Thunberg): Initial findings on multiple environmental stressors in Puget Sound, Washington, 1998. J Shellfish Res 19:353–359
Chiou SH, Yu CW, Lin CW, Pan FM, Lu SF, Lee HJ, Change GG (1995) Octopus S-crystallins with endogenous glutathione S-transferase: cDNA sequence determination, transcription and comparison of structure and kinetic mechanism with authentic enzymes. Biochem J 309:793–800
Choquet G, Soudant P, Lambert C, Nicolas J-L, Paillard C (2003) Reduction of adhesion properties of Ruditapes philippinarum hemocytes exposed to Vibrio tapetis. Dis Aquat Org 57:109–116
Chuang C-C, Wu S-H, Chiou S-H, Chang G-G (1999) Homology modeling of cephalopod lens S-crystallin: a natural mutant of sigma-class glutathione transferase with diminished endogenous activity. Biophys J 76:679–690
Clegg JS, Uhlinger K, Jackson S, Cherr G, Rifkin E, Friedman C (1998) Induced termotolerance and the heat-shock protein 70 family in the Pacific oyster Crassostrea gigas. Mol Mar Biol Biotechnol 7:21–30
Cnaani A (2006) Genetic perspective on stress response and disease resistance in aquaculture. Israeli J Aquaculture 58:375–383
Cuesta R, Laroia G, Schneider R (2000) Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev 14:1460–1470
Cummins S, Nichols A, Amare A, Hummons A, Sweedler J, Nagle G (2004) Characterization of Aplysia rtifact and temptin, two novel water-borne protein pheromones that act in concert with attractin to stimulate mate attraction. J Biol Chem 279:25614–25622
David E, Tanguy A, Pichavant K, Moraga D (2005) Response of the Pacific oysterCrassostrea gigas to hypoxia exposure under experimental conditions. FEBS J 272:5635–5652
David E, Boudry P, Degremont L, Tanguy A, Quere N, Samain J-F, Moraga D (2007) Genetic polymorphism of glutamine synthetase and delta-9 desaturase in families of Pacific oyster Crassostrea gigas and susceptibility to summer mortality. J Exp Marine Biol Ecol 349:272–283
Dégremont L, Ernaude B, Bédier E, Boudry P (2007) Summer mortality of hatchery-reared Pacific oyster (Crassostrea gigas). I. Estimation of genetic parameters for survival and growth. Aquaculture 262:41–53
Doerwald L, van Genesena S, Onnekinka C, Marín-Vinadera L, de Lange F, deonga W, Lubsen N (2006) The effect of αB-crystallin and Hsp27 on the availability of translation initiation factors in heat-shocked cells. Cell Mol Life Sci 63:735–743
Flanagan S, Moseley P, Buettner G (1998) Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett 431:285–286
Foreman H, Fischer AB (1981) Antioxidant defenses. In: Gilbert D (ed) Oxygen and living processes. Springer, New York, pp 235–250
Friedman CS, Estes R, Stokes NA, Burge C, Hargove J, Barber B, Elston R, Burreson E, Reece K (2005) Herpes virus in juvenile Pacific oysters Crassostrea gigas from Tomales Bay, California, coincides with summer mortality episodes. Dis Aquat Org 63:33–41
Garnier M, Labreuche Y, Garcia C, Robert M, Nicolas J-L (2007) Evidence for the involvement of pathogenic bacteria in summer mortalities of the Pacific oyster Crassostrea gigas. Micro Ecol 53:187–196
Gentleman R, Carey V, Bates D, Bolstad B, Dettling M, Dudoi S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Torsten H, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini A, Sawitzki G, Smith C, Smyth G, Tierny L, Yang J, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Bio 5:R80
Greenhalgh C, Alexander W (2004) Suppressors of cytokine signaling and regulation of growth hormone action. Growth Horm IGF Res 3:200–206
Griscourt L, Bonnec G, Boujard D, Mathieu M, Kellner K (2003) Insulin-like system and growth regulation in the Pacific oyster Crassostrea gigas: hrIGF-1 effect on protein synthesis of mantle edge cells and transcription of an homologous insulin receptor-related receptor. Gen Comp Endo 13:44–56
Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguiere C, Girardot AL, Garnier J, Hoareau A, Bachere E, Escoubas JM (2003) Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene 303:139–145
Guillou F, Mitta G, Galinier R, Coustau C (2007) Identification and transcription of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Dev Comp Immunol 31:657–671
Hamdoun AM, Cheney DP, Cherr GN (2003) Phenotypic plasticity of HSP70 and HSP70 gene transcription in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol Bull 205:160–169
Hawkins AJS (1991) Protein turnover: a functional appraisal. Funct Ecol 5:222–233
Hawkins AJS, Day A (1996) The metabolic basis of genetic differences in growth efficiency among marine animals. J Exp Mar Biol Ecol 203:93–115
Hawkins AJS, Wilson IA, Bayne BL (1987) Thermal responses reflect protein turnover in Mytilus edulis L. Func Ecol 1:339–351
Hawkins AJS, Rusin J, Bayne BL, Day A (1989) The metabolic/physiological basis of genotype-dependent mortality during copper exposure in Mytilus edulis. Mar Env Res 28:253–257
Hégaret H, Wikfors G, Soudant P (2003) Flow cytometric analysis of hemocytes from oysters, Crassostrea virginica, subjected to a sudden temperature evelation II. Hemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J Exp Mar Biol Ecol 293:249–265
Hégaret H, Wikfors GH, Soudant P, Delaporte M, Alix JH, Smith BC, Dixon MS, Quere C, Le Cox JR, Paillard C, Moal J, Samain J-F (2004) Immunological competence of eastern oysters, Crassostrea virginica, fed with different microalgal diets and challenged with a temperature elevation. Aquaculture 234:541–560
Hirota K, Matsui M, Murata M, Takashima Y, Cheng F, Itoh T, Fukuda K, Junji Y (2000) Nucleoredoxin, glutaredoxin, and thioredoxin differentially regulate NF-кB, AP-1, and CREB activation in HEK293 cells. Biochem Biophys Res Comm 274:177–182
Hochacka P, Somero G (2002) Biochemical adaptation, 2nd edn. Oxford, New York
Huvet A, Herpin A, Degremont L, Labreuche Y, Samain J-F, Cunningham C (2004) The identification of genes from the oyster Crassostrea gigas that are differentially expressed in progeny exhibiting opposed susceptibility to summer mortality. Gene 343:211–220
Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential transcription. Bioinformatics 18(Suppl. 1):S96–S104
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comp Graphical Stat 5(3):299–314
Jenny M, Chapman R, Mancia A, Chen Y-A, McKillen D, Trent H, Lang P, Escoubas J-M, Bachere E, Boulo V, Liu J, Cunningham C, Cupit P, Tanguy A, Guo X, Moraga D, Boutet I, Huvet A, De Guise S, Almeida J, Warr G (2007) A cDNA Microarray for Crassostrea virginica and C. gigas. Mar Biotech 9:577–591
Johansson M, Lind M, Holmblad T, Thörnqvist P, Söderhäll K (1995) Peroxinectin, a novel cell adhesion protein from crayfish blood. Biochem Biophys Res Com 216:1079–1087
Kalra SP, Kalra PS (2004) NPY an endearing journey in search of neurochemical on/off switch for appetite, sex, and reproduction. Peptides 25:465–471
Kang Y-S, Kim Y-M, Park K-I, Cho S, Choi K-S, Cho M-C (2006) Analysis of EST and lectin transcriptions in hemocytes of Manila clams (Ruditapes philippinarum) (Bivalvia: Mollusca) infected with Perkinsus oleseni. Dev Comp Immunol 30:1119–1131
Kanost M (1999) Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol 23:291–301
Labrueche Y, Lambert C, Soudant P, Boulo V, Huvet A, Nicolas J-L (2006) Cellular and molecular hemocyte reponses to the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes Infect 8:2715–2724
Lacoste A, Jalabert F, Malham S, Cueff A, Gélébeart F, Cordevant C, Lange M, Poulet S (2001) A Vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the bay of Morlaix (North Brittany, France). Dis Aquat Org 46:139–145
Lambert C, Soudant P, Dégremont L, Delaporte M, Moal J, Boudry P, Jean F, Huvet A, Samain JF (2007) Hemocyte characteristics in families of oysters, Crassotrea gigas, selected for differential survival during summer mortality and reared in three sites. Aquaculture 270:276–288
Langdon C, Evans F, Jacobson D, Blouin M (2003) Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture 220:227–244
Levroney E, Aguilar H, Fulcher J, Kohatsu L, Pace K, Pang M, Gurney K, Baum L, Benhur L (2005) Novel innate immune functions for galectin-1: Galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol 175:413–420
Lindquist S (1986) The heat shock response. Ann Rev Biochem 55:1151–1191
Li Y, Qin J, Abbott C, Li X, Benkendorff (2007) Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters. Am J Physiol Regul Integr Comp Physiol 293:2353–2362
Li Y, Qin J, Abbott C, Li X, Benkendorff (2009) Spawning-dependent stress response to food deprivation in Pacific oyster Crassostrea gigas. Aquaculture 286:309–317
Liu CH, Cheng W, Chen JC (2005) The peroxinectin of white shrimp Litopenaeus vannamei is synthesized in the semi-granular and granular cell, and its transcription is upregulated with Vibrio alginolyticus infection. Fish Shell Immunol 18:431–444
Liu CH, Yeh SP, Hsu PY, Cheng W (2007) Peroxinectin gene transcription of the giant freshwater prawn Machobranchium rosenbergii under intrinsic, immunostimulant, and chemotherapeutant influences. Fish Shell Immunol 22:408–417
Ma P, Castillo-David C, Zhong W, Liu J (2006) A data-driven clustering method for time course gene transcription data. Nuc Ac Res 34:1261–1269
Martin C, Oh C-S, Jiang Y (2006) Regulation of long chain fatty acid synthesis in yeast. Biochim Biophys Acta 1771:271–285
Meistertzheim A-L, Tanguy A, Moraga D, Thebault M-T (2007) Identification of differentially expressed genes of the Pacific oyster Crassostrea gigas exposed to prolonged thermal stress. FEBS J 24:6392–6402
Montagnani C, Tirape A, Boulo V, Escobas JM (2005) The two Cg-timp mRNAs expressed in oyster hemocytes are generated by two gene families and differentially expressed during ontogenesis. Dev Comp Immunol 29:831–839
Ntambi J (1999) Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40:1549–1558
Oliver JL, Gaffney PM, Allen SK, Faisal M, Kaattari SL (2000) Protease inhibitory activity in selectively bred families of Eastern oysters. J Aquat Anim Health 12:136–145
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Perillo N, Marcus M, Baum L (1998) Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 76:402–412
Rabinovich G, Rubinstein N, Fainboim (2002) Unlocking the secrets of galectins: a challenge at the frontier of glyco-immunology. J Leukoc Bio 71:741–752
Ramakers C, Ruijter JM, Deprez RH, Moorman AF (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66
Rincón G, Young AE, Bannasch DL, Medrano JF (2007) Charaterization of variation in the canine suppressor of cytokine signaling-2 (SOCS-2) gene. Genet Mol Res 6:144–151
Rinne R, Saukko P, Järvinen M, Lehesjoki A-E (2002) Reduced cystatin B activity correlates with enhanced cathepsin activity in progressive myoclonus epilepsy. Ann Med 34:380–385
Romestead B, Corbier F, Roch P (2002) Protease inhibitors and haemagglutins associated with resistance to the protozoan parasite, Perkinsus marinus, in the Pacific oyster, Crassostrea gigas. Parasitol 125:323–329
Rothschild M, Ruvinsky A (2007) Marker-assisted selection for aquaculture species. In: Liu Z (ed) Aquaculture genome technologies. Blackwell, Ames, pp 199–215
SAS Institute Inc. (1999) SAS/STAT User’s Guide. Version 8. SAS Institute, Cary
Samain JF, Dégremont L, Soletchnik P, Haure J, Bédier E, Ropert M, Moal J, Huvet A, Bacca H, Van Wormhoudt A, Delaporte M, Costil K, Pouvreau S, Lambert C, Boulo V, Soudant P, Nicolas JL, Le Roux F, Renault T, Gagnaire B, Geret F, Boutet I, Burgeot T, Boudry P (2007) Genetically based resistance to summer mortality in the Pacific oyster (Crassostrea gigas) and its relationship with physiological, immunological characteristics and infection processes. Aquaculture 268:227–243
Sauvage C, Bierne N, Boudry P (2007) Single nucleotide polymorphisms and their relationship to codon usage bias in the Pacific oyster Crassostrea gigas. Gene 406:13–22
Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledlius C, Jacobsen P, Zechner R, Zimmermann R (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241
Soletchnik P, Le Moine O, Faury N, Razet D, Geairon P, Goulletquer P (1999) Summer mortality of the oyster in the Bay Marennes-Oleron: spatial variability of environment and biology using a geographical information system (GIS). Aquat Living Resou 12:131–144
Soletchnik P, Lambert C, Costil K (2005) Summer mortality of Crassostrea gigas (Thunberg) in relation to rearing environment conditions. J Shellfish Res 24:197–207
Soletchnik P, Faury N, Goulletquer P (2006) Seasonal changes in carbohydrate metabolism and its relationship with summer mortality of Pacific oyster Crassostrea gigas (Thunberg) in Marennes-Oléron bay (France). Aquaculture 252:328–338
Soletchnik P, Ropert M, Mazuie J, Fleury P, Le Coz F (2007) Relationships between oyster mortality patterns and environmental data from monitoring databases along the coasts of France. Aquaculture 271:384–400
Storch J, Thumser A (2000) The fatty acid transport and function of fatty acid-binding proteins. Biochim Biophys Act 1486:28–44
Storey K (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Storey JD (2002) A direct approach to false discovery rate. J R Statist Soc B 64:479–498
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Tanguy A, Bierne N, Saaverdra C, Pina B, Bachere E, Kube M, Bazin E, Bonhomme F, Boudry P, Boulo V, Boutet I, Cancela L, Doussat C, Favrel P, Huvet A, Jarque S, Jollivet D, Klages S, Lapegue S, Leite R, Moal J, Moraga D, Reinhardt R, Samain J-F, Zouros E, Canario A (2008) Increasing genomic information in bivalves through new EST collections in four species: development of new genetic markers for environmental studies and genome evolution. Gene 408:27–36
Taris N, Lang P, Camara M (2008) Sequence polymorphism can produce serious artifacts in real-time PCR assays: hard lessons from Pacific oysters. BMC Genomcs 9:234
Tasumi S, Vasta G (2007) A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J Immunol 179:3086–3098
Tensen CP, Cox KJ, Burke JF, Leurs R, van der Schors RC, Geraerts WP, Vreuhdenhil E, Heerikhuizen H (1998) Molecular cloning and characterization of an invertebrate homologue of a neuropeptide Y receptor. Eur J Neurosci 10:3409–3416
Tibile R, Singh H (2003) Larval rearing and spat production of edible oyster Crassostrea gryphoides. Aquaculture Res 34:785–792
Tomarev S, Piatgorrsky J (1996) Lens crystallins of invertebrates. Diversity and recruitment from detoxification enzymes and novel proteins. Eur J Biochem 235:449–465
Tsvetkova N, Horváth I, Török Z, Wolkers Wm Balogi Z, Shigapova N, Crowe L, Tablin F, Vierling E, Crowe J, Vigh L (2002) Small heat-shock proteins regulate lipid polymorphism. Proc Natl Acad Sci USA 99:13504–13509
Ulrich K (1995) Comparitive animal biochemistry. Springer, New York, p 248
Vergote D, Bouchut A, Sautiere PE, Roger E, Galinier R, Rognon A, Coustau C, Slazet M, Mitta G (2005) Characterization of proteins differentially present in the plasma of Biomphalaria glabrata susceptible or resistant to Echinostoma caproni. Internat Jour Parasit 32:215–224
Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chemico-Biological Interact 167:219–226
Wang K, Spector A (2000) α-Crystallin prevents irreversible protein denaturation and acts cooperatively with other heat-shock proteins to renature the stabilized partially denatured protein in an ATP-dependent manner. Eur J Biochem 267:4705–4712
Woessner J (1998) The matrix metalloproteinase family. In: Parks WC, Mecham RP (eds) Matrix metalloproteinases. Academic, San Diego, pp 1–14
Xue QG, Waldrop GL, Chey KL, Itoh N, Ogawa M, Cooper R, Losso J, La Peyre JF (2006) A novel slow-tight binding serine protease inhibitor from eastern oyster (Crassostrea virginica) plasma inhibits perkinsin, the major extracellular protease of the oyster protozoan parasite Perkinsus marinus. Comp Biochem Physiol B 145:16–26
Yamaura K, Takahashi K, Suzuki T (2008) Identification and tissue transcription analysis of C-type lectin and galectin in Pacific oyster, Crassostrea gigas. Comp Biochem Phys B 149:168–175
Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signaling and immune regulation. Nat Rev Immu 7:454–465
Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186
Ziegler G, Paynter K, Fisher D (2002) Matrix metalloproteinase-like activity from hemocytes of the eastern oyster, Crassostrea virginica. Comp Biochem Physiol B 131:361–370
Zhao R, Houry W (2007) Molecular interaction network of the Hsp90 chaperone system. In: Csermely P, Vigh L (eds) Molecular aspects of the stress response: Chaperones, membranes, and networks (Advances in experimental medicine and biology 594). Springer, New York, pp 27–36
Acknowledgements
Funding for this research was provided by the Oyster Disease Research Program, NOAA agreement no. NA16RG1039, Sea Grant Project SAQ-08-NSI. Additional funding was provided by the Lylian Brucefield Scholarship and the Mamie Markham Award. We thank A. Barton, F. Evans, D. Mosher, and D. Stick for rearing the oyster families used during this experiment and for assistance during the heat-shocking experiments. We thank Oregon Oyster Farms, Newport, Oregon for the generous use of their facilities. We thank R. Chapman, Y. A. Chen, M. Cook, P. Cupit, P. Gross, M. Lundqvist, D. MacKillan, A. Mancia, N. Taris, H. Trent, and G. Warr for technical assistance and support during the hybridization of the microarrays and during data analysis. We thank W. Huber and W. Zhong for their advice during data analysis using vsn and SSClust, respectively. We thank A. Huvet, N. Taris, and two anonymous reviewers for thoughtful review of this manuscript. Any use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the image is a link to a high resolution version.
Supplementary Figure 1
Raw output for 95% confidence intervals for ESTs in which transcription level differed among sampling times in gill of heat-shocked (40°C, 1 h) oysters before and at 1, 3, 6, and 24 h after heat shock. Data were clustered using the program SSClust. The vsn-transformed data for each sampling time are presented relative to the average for all sampling times for a given EST. (GIF 129 kb)
Supplementary Figure 2
Average vsn-transformed signal (±SEM) for ESTs that were not studied using real-time quantitative PCR. Gill samples were collected before and at 1, 3, 6, and 24 h after heat shock (40°C, 1 h), and RNAs from those samples were hybridized to cDNA microarrays. In each plot, families with low survival (<29%) after heat shock are represented by hatched bars and those with high survival (>64%) are represented by white bars. Each bar represents nine individuals from each of two individual families of the specified survival type. (JPG 2.67 MB)
Supplementary Figure 3
Average vsn-transformed signal (±SEM) for ESTs that were not studied using real-time quantitative PCR. Gill samples were collected before and at 1, 3, 6, and 24 h after heat shock (40°C, 1 h), and RNAs from those samples were hybridized to cDNA microarrays. In each plot, families with low survival (<29%) after heat shock are represented by hatched bars and those with high survival (>64%) are represented by white bars. Each bar represents nine individuals from each of two individual families of the specified survival type. (JPG 2.52 MB)
Rights and permissions
About this article
Cite this article
Lang, R.P., Bayne, C.J., Camara, M.D. et al. Transcriptome Profiling of Selectively Bred Pacific Oyster Crassostrea gigas Families that Differ in Tolerance of Heat Shock. Mar Biotechnol 11, 650–668 (2009). https://doi.org/10.1007/s10126-009-9181-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9181-6