Abstract
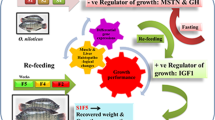
Circadian clock genes and myogenic factors are tightly integrated to influence muscle growth upon dietary deprivation in animals. In this study, we reported that upon short-term fasting of Nile tilapia juveniles for 7 and 15 days, the growth of the fish stagnated and the size of muscle fibers decreased. To reveal the molecular mechanisms of how starvation affects fish muscle growth, we analyzed the rhythmic expression of circadian clock genes and myogenic factors. After 7 and 15 days of fasting treatment, the muscle tissues were collected for 24 h (at zeitgeber times ZT0, ZT3, ZT6, ZT9, ZT12, ZT18, ZT21, and ZT24) from tilapia juveniles. Among the 27 clock genes, the expression of cyr1b, nr1d1, per1, clocka, clockb, ciarta, and aanat2 displayed a daily rhythmicity in normal daily cycle, while arntl2, cry1a, cry1b, npas2, nr1d2b, per2, per3, rorαb, clocka, clockb, nfil3, cipca, and cipcb exhibited daily rhythmicity in the fasting fish muscles. The transcript levels of clockb showed moderate positive correlation with the aanat2, ciarta, cry1b, and nr1d1 in the muscle tissue of normally fed Nile tilapia juvenile. In comparison of the two treatment modes, the expression levels of clocka, clockb, and cry1b showed the rhythmicity, but clockb expression was significantly decreased and the acrophase had shifted. The transcript levels of fbxo32 and pdk4 had either moderate or strong positive correlations with other daily expression of clock genes except arntl2 in the muscle after 7-day fasting. The expressions of myogenic regulatory factors were also either upregulated or downregulated. These observations demonstrated that dietary starvation might affect fish muscle growth by modulating the differential expression of circadian clock genes and myogenic factors. Thus, our work provides a better understanding of the molecular mechanism of dietary starvation on fish growth and may provide dietary administration in aquiculture.








Similar content being viewed by others
References
Amaral IP, Johnston IA (2012) Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. Am J Physiol Regul Integr Comp Physiol 302:26
Andrews JL et al (2010a) CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A 107:19090
Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA (2010b) CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A 107:19090–19095
Beaver LM, Rush BL, Gvakharia BO, Giebultowicz JM (2003) Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster. J Biol Rhythm 18:463–472
Bortolato B, Berk M, Maes M, McIntyre RS, Carvalho AF (2016) Fibromyalgia and Bipolar Disorder: Emerging Epidemiological Associations and Shared Pathophysiology. Curr Mol Med 16:119–136
Bugge A et al (2012) Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev 26:657–667
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Cahill GM (2014) Clock mechanisms in zebrafish. Cell Tissue Res 309:27–34
Chong SW, Nguyet LM, Jiang YJ, Korzh V (2007) The chemokine Sdf-1 and its receptor Cxcr4 are required for formation of muscle in zebrafish. BMC Dev Biol 7:54
Costa LS, Serrano I, Sanchez-Vazquez FJ, Lopez-Olmeda JF (2016) Circadian rhythms of clock gene expression in Nile tilapia (Oreochromis niloticus) central and peripheral tissues: influence of different lighting and feeding conditions. J Comp Physiol B 186:775–785
Crumbley C, Wang Y, Kojetin DJ, Burris TP (2010) Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem 285:35386–35392
Cuesta IH, Lahiri K, Lopez-Olmeda JF, Loosli F, Foulkes NS, Vallone D (2014) Differential maturation of rhythmic clock gene expression during early development in medaka (Oryzias latipes). Chronobiol Int 31:468–478
Duan C, Hirano T (1992) Effects of insulin-like growth factor-I and insulin on the in-vitro uptake of sulphate by eel branchial cartilage: evidence for the presence of independent hepatic and pancreatic sulphation factors. J Endocrinol 133:211–219
Emery P, Reppert SM (2004) A rhythmic Ror. Neuron 43:443–446
Falcon J (1999) Cellular circadian clocks in the pineal. Prog Neurobiol 58:121–162
Falcon J, Migaud H, Munoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 165:469–482
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Gutierrez J, Carrillo M, Zanuy S, Planas J (1984) Daily rhythms of insulin and glucose levels in the plasma of sea bass Dicentrarchus labrax after experimental feeding General & Comparative Endocrinology 55:393–397
Khan ZA, Yumnamcha T, Rajiv C, Sanjita Devi H, Mondal G, Devi SD, Bharali R, Chattoraj A (2016) Melatonin biosynthesizing enzyme genes and clock genes in ovary and whole brain of zebrafish (Danio rerio): differential expression and a possible interplay. Gen Comp Endocrinol 233:16–31
Kim HJ, Lee HR, Seo JY, Ryu HG, Lee KH, Kim DY, Kim KT (2017) Heterogeneous nuclear ribonucleoprotein A1 regulates rhythmic synthesis of mouse Nfil3 protein via IRES-mediated translation. Sci Rep 7:42882
Labrecque MP, Prefontaine GG, Beischlag TV (2013) The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces. Curr Mol Med 13:1047–1065
Lazado CC, Kumaratunga HP, Nagasawa K, Babiak I, Giannetto A, Fernandes JM (2014a) Daily rhythmicity of clock gene transcripts in atlantic cod fast skeletal muscle. PLoS One 9:e99172
Lazado CC, Nagasawa K, Babiak I, Kumaratunga HP, Fernandes JM (2014b) Circadian rhythmicity and photic plasticity of myosin gene transcription in fast skeletal muscle of Atlantic cod (Gadus morhua). Mar Genomics 18:21–29
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Mazzoccoli G et al (2013) Circadian transcriptome analysis in human fibroblasts from hunter syndrome and impact of iduronate-2-sulfatase treatment. BMC Med Genet 6:1755–8794
McCarthy JJ et al (2007) Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31:86–95
McStay E, Migaud H, Vera LM, Sánchez-Vázquez FJ, Davie A (2014) Comparative study of pineal clock gene and AANAT2 expression in relation to melatonin synthesis in Atlantic salmon (Salmo salar) and European seabass (Dicentrarchus labrax). Comp Biochem Physiol A Mol Integr Physiol 169:77–89
Naidu PS, Ludolph DC, To RQ, Hinterberger TJ, Konieczny SF (1995) Myogenin and MEF2 function synergistically to activate the MRF4 promoter during myogenesis. Mol Cell Biol 15:2707–2718
Nakao R, Shimba S, Oishi K (2017) Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci Rep 7:017–03119
Ohta Y et al (2017) Clock gene dysregulation induced by chronic ER stress disrupts beta-cell function. eBioMedicine 18:146–156
Panda S et al (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–320
Plautz JD, Kaneko M, Hall JC, Kay SA (1997) Independent photoreceptive circadian clocks throughout Drosophila. Science 278:1632–1635
Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM (2006) Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol 2:10
Shavlakadze T, Anwari T, Soffe Z, Cozens G, Mark PJ, Gondro C, Grounds MD (2013) Impact of fasting on the rhythmic expression of myogenic and metabolic factors in skeletal muscle of adult mice. Am J Physiol Cell Physiol 305:17
Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485:62–68
Thissen JP, Ketelslegers JM, Underwood LE (1994) Nutritional regulation of the insulin-like growth factors. Endocr Rev 15:80–101
Vatine G, Vallone D, Gothilf Y, Foulkes NS (2011) It’s time to swim! Zebrafish and the circadian clock. FEBS Lett 585:1485–1494
Velarde E, Haque R, Iuvone PM, Azpeleta C, Alonso-Gomez AL, Delgado MJ (2009) Circadian clock genes of goldfish Carassius auratus: cDNA cloning and rhythmic expression of period and cryptochrome transcripts in retina, liver, and gut. J Biol Rhythm 24:104–113
Vera LM, Negrini P, Zagatti C, Frigato E, Sanchez-Vazquez FJ, Bertolucci C (2013) Light and feeding entrainment of the molecular circadian clock in a marine teleost (Sparus aurata). Chronobiol Int 30:649–661
Wang H (2008a) Comparative analysis of period genes in teleost fish genomes. J Mol Evol 67:29–40
Wang H (2008b) Comparative analysis of teleost fish genomes reveals preservation of different ancient clock duplicates in different fishes. Mar Genomics 1:69–78
Watanabe WO, Losordo TM, Fitzsimmons K, Hanley F (2002) Tilapia production systems in the Americas: technological advances, trends, and challenges. Rev Fish Sci 10:465–498
Woods IG, Wilson C, Friedlander B, Chang P, Reyes DK, Nix R, Kelly PD, Chu F, Postlethwait JH, Talbot WS (2005) The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res 15:1307–1314
Wu P, Li YL, Cheng J, Chen L, Zhu X, Feng ZG, Zhang JS, Chu WY (2016) Daily rhythmicity of clock gene transcript levels in fast and slow muscle fibers from Chinese perch (Siniperca chuatsi). BMC Genomics 17:1008
Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219–16224
Zhao WN, Malinin N, Yang FC, Staknis D, Gekakis N, Maier B, Reischl S, Kramer A, Weitz CJ (2007) CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol 9:268–275
Funding
This study was supported by the National Natural Science Foundation of China (No. 31472256, 31230076 and No.31572592) and Collaborative Innovation Center for Efficient and Health Production of Fisheries, Hunan Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, P., Chu, W., Liu, X. et al. The Influence of Short-term Fasting on Muscle Growth and Fiber Hypotrophy Regulated by the Rhythmic Expression of Clock Genes and Myogenic Factors in Nile Tilapia. Mar Biotechnol 20, 750–768 (2018). https://doi.org/10.1007/s10126-018-9846-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9846-0