Abstract
Schizophrenia (SCZ) is one of the brain disorders which affects the thinking and behavioral skills of patients. This disorder comes along with an overproduction of kynurenic acid in the cerebrospinal fluid and the prefrontal cortex of SCZ patients. In this study, marine bacterial compounds were screened for their suitability as antagonists against human kynurenine aminotransferase (hKAT-1) which causes the synthesis of kynurenic acid downstream which ultimately causes the SCZ disorder according to the kynurenic hypothesis of SCZ. The marine actinobacterial compound bonactin shows more promising results than other tested marine compounds such as the histamine H2 blocker famotidine and indole-3-acetic acid (IAC) from docking and in silico toxicological studies carried out here. The obtained results of the Grid-based Ligand Docking with Energetics (Glide) scores of extra-precision (XP) Glide against the target protein hKAT-1 on IAC, famotidine, and bonactin were − 6.581, − 6.500 and − 7.730 kcal/mol where Glide energies were − 29.84, − 28.391, and − 47.565 kcal/mol, respectively. Bonactin is known as an antibacterial and antifungal compound being extracted from a marine Streptomyces sp. Comparing tested compounds against the drug target hKAT-1, bonactin alone showed the best Glide score and Glide energy on the target protein hKAT-1.








Similar content being viewed by others
Abbreviations
- IAC :
-
indole-3-acetic acid
- XP :
-
extra-precision
- hKAT :
-
human kynurenine aminotransferase
- kcal/mol :
-
kilocalories/mole
- Glide :
-
grid-based ligand docking with energetics; GEMODEL/GEVDW (depending on authors)
- RBA :
-
relative binding affinity
- IMN :
-
Istituto Mario Negri
- SCZ :
-
schizophrenia
- CNS :
-
central nervous system
- KYNA :
-
kynurenic acid
- CSF :
-
cerebrospinal fluid
- BBB :
-
blood–brain barrier
- AIDS :
-
acquired immune deficiency syndrome
- SP :
-
standard precision
- HTVS :
-
high-throughput virtual screening
References
Akladios FN, Nadvi NA, Park J, Hanrahan JR, Kapoor V, Gorrell MD, Bret Church W (2012) Design and synthesis of novel inhibitors of human kynurenine aminotransferase-I. Bioorg Med Chem Lett 22(4):1579–1581
Alireza N, Guanchen S, Gayan S, Bret Church W (2016) Kynurenine aminotransferase isozyme inhibitors: a review. Int J Mol Sci 17(1):1–22
American Psychiatric Association (2013) DSM-5®-Diagnostic and statistical manual of mental disorders, 5th edn.
Aouiche A, Bijani C, Zitouni A, Mathieu F, Sabaou N (2014) Antimicrobial activity of saquayamycins produced by Streptomyces spp. PAL114 isolated from a Saharan soil. J Mycol Med 24(2):e17–e23
Aoyagi T, Hatsu M, Imada C, Naganawa H, Okami Y, Takeuchi T (1992) Pyrizinostatin: a new inhibitor of pyroglutamyl peptidase. J Antibiot (Tokyo) 45(11):1795–1796
Asolkar RN, Jensen PR, Kauffman CA, Fenical W (2006) Daryamides A–C, weakly cytotoxic polyketides from a marine-derived actinomycete of the genus Streptomyces strain CNQ-085. J Nat Prod 69(12):1756–1759
Asolkar RN, Schröder D, Heckmann R, Lang S, Wagner-Döbler I, Laatsch H (2004) Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1+. J Antibiot (Tokyo) 57(1):17–23
Berman HM, Kleywegt GJ, Nakamura H, Markley JL (2014) The Protein Data Bank archive as an open data resource. J Comput Aided Mol Des 28(10):1009–1014
Bister B, Bischoff D, Ströbele M, Riedlinger J, Reicke A, Wolter F, Bull AT, Zähner H, Fiedler HP, Süssmuth RD (2004) Abyssomicin C–A polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew Chem Int Ed Engl 43(19):2574–2576
Brugnoli R, Rapinesi C, Kotzalidis GD, Marcellusi A, Mennini FS, de Filippis S, Carrus D, Ballerini A, Francomano A, Ducci G, del Casale A, Girardi P (2016) Model of management (Mo.Ma) for the patient with schizophrenia: crisis control, maintenance, relapse prevention, and recovery with long-acting injectable antipsychotics (LAIs). Riv Psichiatr 51:47–59
Cassano A, Raitano G, Mombelli E, Fernández A, Cester J, Roncaglioni A, Benfenati E (2014) Evaluation of QSAR models for the prediction of ames genotoxicity: a retrospective exercise on the chemical substances registered under the EU REACH regulation. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 32(3):273–298
Citrome L (2017) A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother 13:1545–1573
Cho JY, Kwon HC, Williams PG, Jensen PR, Fenical W (2006a) Azamerone, a terpenoid phthalazinone from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). Org Lett 8(12):2471–2474
Cho JY, Kwon HC, Williams PG, Kauffman CA, Jensen PR, Fenical W (2006b) Actinofuranones A and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). J Nat Prod 69(3):425–428
Ding L, Qin S, Li F, Laatsch H (2008) Novel chacolmycins possessing an amino sugar unit isolated from the marine Streptomyces sp. M491. J Biotechnol 136:S579
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313(1–2):96–98
Floris M, Albanese F, Medda R, Benfenati E (2016) Comprehensive safety evaluation of the chemicals with human and environmental relevance
Fraìczek T, Siwek A, Paneth P (2013) Assessing molecular docking tools for relative biological activity prediction: a case study of triazole HIV-1 NNRTIs. J Chem Inf Model 53(12):3326–3342
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49(21):6177–6196
Gattaz WF, Waldmeier P, Beckmann H (1982) CSF monoamine metabolites in schizophrenic patients. Acta Psychiatr Scand 66(5):350–360
Gayan S, Jayawickrama NA, Sun G, Gorrell MD, Church BW (2017) Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci Rep 7(17559):1–11
Glide user manual 5.5 Copyright © (2009) Schrödinger, LLC. New York, NY, MCPRO - trademark of William L. Jorgensen. Desmond - trademark of D. E. Shaw Research. Desmond - with the permission of D. E. Shaw Research. Schrödinger Press. Available from: https://isp.ncifcrf.gov/files/isp/uploads/2010/07/gli55_user_manual.pdf
Gros M, Petrović M, Barceló D (2006) Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 70(4):678–690
Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R (2007) Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem 102(1):103–111
Guillemin GJ, Kerr SJ, Brew BJ (2005) Involvement of quinolinic acid in aids dementia complex. Neurotox Res 7(1–2):103–123
Gupta J, Kulshreshtha M (2017) Memory impairment with reference to Alzheimer’s disease: an update. Int J Nutr Pharmacol Neurol Dis 7(3):45
Han Q, Robinson H, Cai T, Tagle DA, Li J (2009) Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K. J Med Chem 52(9):2786–2793
Hert MD, Dekker JM, Wood D, Kahl KG, Moller H-J (2009) Cardiovascular disease and diabetes in people with severe mental illness. Rev Psiquiatr Salud Ment 2(1):49–59
Iannitelli A, Quartini A, Tirassa P, Bersani G (2017) Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev 80:414–442
Jablensky A (2010) The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues Clin Neurosci 12(3):271–287
Jensen GE, Niemelä JR, Wedebye EB, Nikolov NG (2008) QSAR models for reproductive toxicity and endocrine disruption in regulatory use—a preliminary investigation. SAR QSAR Environ Res 19(7–8):631–641
Jensen PR, Williams PG, Oh DC, Zeigler L, Fenical W (2007) Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol 73(4):1146–1152
Jeong SY, Shin HJ, Kim TS, Lee HS, Park SK, Kim HM (2006) Streptokordin, a new cytotoxic compound of the methylpyridine class from a marine-derived Streptomyces sp. KORDI-3238. J Antibiot (Tokyo) 59(4):234–240
Kanoh K, Matsuo Y, Adachi K, Imagawa H, Nishizawa M, Shizuri Y (2005) Mechercharmycins A and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J Antibiot (Tokyo) 58(4):289–292
Kiszkiel I, Starczewska B, Karpińska J (2012) New extraction procedures for isolation of famotidine from aqueous samples. Int J Environ Anal Chem 92(6):714–728
Kock I, Maskey RP, Biabani MAF, Helmke E, Laatsch H (2005) 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot (Tokyo) 58(8):530–534
Kubinyi H (1998) Structure-based design of enzyme inhibitors and receptor ligands. Curr Opin Drug Discov Devel 1(1):4–15
Kwon HC, Kauffman CA, Jensen PR, Fenical W (2006) Marinomycins A-D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora”. J Am Chem Soc 128(5):1622–1632
Li F, Maskey RP, Qin S, Sattler I, Fiebig HH, Maier A, Zeeck A, Laatsch H (2005) Chinikomycins A and B: isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045. J Nat Prod 68(3):349–353
Liu R, Zhu T, Li D, Gu J, Xia W, Fang Y, Liu H, Zhu W, Gu Q (2007) Two indolocarbazole alkaloids with apoptosis activity from a marine-derived actinomycete Z 2 039-2. Arch Pharm Res 30(3):270–274
Macherla VR, Liu J, Bellows C, Teisan S, Nicholson B, Lam KS, Potts BCM (2005) Glaciapyrroles A, B, and C, pyrrolosesquiterpenes from a Streptomyces sp. isolated from an Alaskan marine sediment. J Nat Prod 68(5):780–783
Maskey RP, Helmke E, Kayser O, Fiebig HH, Maier A, Busche A, Laatsch H (2004) Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J Antibiot (Tokyo) 57(12):771–779
Maskey RP, Helmke E, Laatsch H (2003) Himalomycin A and B: isolation and structure elucidation of new fridamycin type antibiotics from a marine Streptomyces isolate. J Antibiot (Tokyo) 56(11):942–949
Meng X-Y, Zhang H-X, Mezei M, Cui M (2011) Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des 7(2):146–157
Meskanen K, Ekelund H, Laitinen J, Neuvonen PJ, Haukka J, Panula P, Ekelund J (2013) A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia. J Clin Psychopharmacol 33(4):472–478
Mitchell SS, Nicholson B, Teisan S, Lam KS, Potts BCM (2004) Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J Nat Prod 67(8):1400–1402
Nematollahi A, Gayan GS, Jayawickrama G, Church WB (2016) Kynurenine aminotransferase isozyme inhibitors: a review. Int J Mol Sci 17:946
Palmer CL, Cotton L, Henley JM (2005) The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev 57(2):253–277
Passera E, Campanini B, Rossi F, Casazza V, Rizzi M, Pellicciari R, Mozzarelli A (2011) Human kynurenine aminotransferase II—reactivity with substrates and inhibitors. FEBS J 278(11):1882–1900
Qian H, Howard R, Tao C, Danilo A, Tagle JL (2009) Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol 1(1):784–793
Quinti L, Dayalan Naidu S, Träger U, Chen X, Kegel-Gleason K, Llères D, Connolly C, Chopra V, Low C, Moniot S, Sapp E, Tousley AR, Vodicka P, Van Kanegan MJ, Kaltenbach LS, Crawford LA, Fuszard M, Higgins M, Miller JRC, Farmer RE et al (2017) KEAP1-modifying small molecule reveals muted NRF2 signaling responses in neural stem cells from Huntington’s disease patients. Proc Natl Acad Sci 114(23):E4676–E4685
Renner MK, Shen YC, Cheng XC, Jensen PR, Frankmoelle W, Kauffman CA, Fenical W, Lobkovsky E, Clardy J (1999) Cyclomarins A–C, new antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.). J Am Chem Soc 121(49):11273–11276
Rissdörfer M, Venugopala KN, Nayak SK, Badea FD (2014) Physicochemical, crystallography and DFT calculations on biologically active dihydropyrimidine analogues. UPB Sci Bull Ser B Chem Mater Sci 76(3):75–88
Romero F, Espliego F, Pérez Baz J, García de Quesada T, Grávalos D, de la Calle F, Fernández-Puentes JL (1997) Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot (Tokyo) 50(9):734–737
Roncaglioni A, Piclin N, Pintore M, Benfenati E (2008) Binary classification models for endocrine disrupter effects mediated through the estrogen receptor. SAR QSAR Environ Res 19:697–733
Rossi F, Han Q, Li J, Li J, Rizzi M (2004) Crystal structure of human kynurenine aminotransferase I. J Biol Chem 279(48):50214–50220
Rossi F, Valentina C, Garavaglia S, Sathyasaikumar KV, Schwarcz R, Kojima SI, Okuwaki K, Ono SI, Kajii Y, Rizzi M (2010) Crystal structure-based selective targeting of the pyridoxal 5′-phosphate dependent enzyme kynurenine aminotransferase II for cognitive enhancement. J Med Chem 53(15):5684–5689
Schrödinger LLC (2008) Glide, version 5.0. Schrödinger. LLC, New York. https://www.schrodinger.com/glide
Schumacher RW, Talmage SC, Miller S, Sarris KE, Davidson BS, Goldberg A (2003) Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J Nat Prod 66(9):1291–1293
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50(7):521–530
Schwarcz R (2004) The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 4(1):12–17
Shiono Y, Shiono N, Seo S, Oka S, Yamazaki Y (2002) Effects of polyphenolic anthrone derivatives, resistomycin and hypericin, on apoptosis in human megakaryoblastic leukemia CMK-7 cell line. Zeitschrift fuer Naturforsch - Sect C J Biosci 57(9–10):923–929
Solanki R, Khanna M, Lal R (2008) Bioactive compounds from marine actinomycetes. Indian J Microbiol 48:410–431
Trotta A, Iyegbe C, Forti M, Di Sham PC, Campbell DD, Cherny SS, Mondelli V, Aitchison KJ, Murray RM, Vassos E, Fisher HL (2016) Interplay between schizophrenia polygenic risk score and childhood adversity in first-presentation psychotic disorder: a pilot study. PLoS One 11(9):1–14
WHO (2013) Information sheet—premature death among people with severe mental disorders. http://www.who.int/mental_health/management/info_sheet.pdf
WHO (2018) Schizophrenia: fact sheets. 9 April 2018. http://www.who.int/en/news-room/fact-sheets/detail/schizophrenia
Williams PG, Asolkar RN, Kondratyuk T, Pezzuto JM, Jensen PR, Fenical W (2007) Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J Nat Prod 70(1):83–88
Yu P, Li L, Zhang D, Tagle A, Cai T (2006) Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene 365(1):111–118
Acknowledgements
The authors thank the University Informatics Centre (UIC), High Performance Computing Facility (HPC) and Bharathidasan University, India, DST-PURSE (Grant No. SR/FT/LS-113/2009), for providing the Schrödinger software access. One of the authors, Muthukumar Krishnan, wishes to thank the Department of Science and Technology, Science Engineering Research Board (DST-SERB), New Delhi, India for awarding the National Postdoctoral fellowship (PDF/2017/002213) and the Director, National Institute of Technology (NIT), Tiruchirappalli – 620 015, Tamil Nadu, India.
Funding
The study was supported by the Bharathidasan University, India, DST-PURSE (Grant No. SR/FT/LS-113/2009). H.-U. Dahms was supported by a grant from the Research Center of Environmental Medicine, Kaohsiung Medical University (KMU), Taiwan (R-1053011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No animals/humans were used for this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig S1
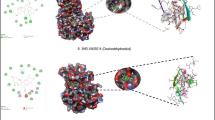
Crystal Structure of Human Kynurenine Aminotransferase I in the complex with Indole-3-acetic Acid (PDB ID-3FVU). Snapshot taken by using NGL 3D viewer. (PNG 1695 kb)
Fig S2
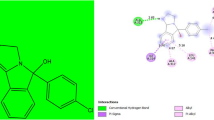
Molecular structures of chemicals. (a) IAC, (b) famotidine and (c) bonactin. ChemDraw was used to draw the chemical structures, available from http://scistore.cambridgesoft.com/. (PNG 180 kb)
Rights and permissions
About this article
Cite this article
Thiyagarajamoorthy, D.K., Arulanandam, C.D., Dahms, HU. et al. Marine Bacterial Compounds Evaluated by In Silico Studies as Antipsychotic Drugs Against Schizophrenia. Mar Biotechnol 20, 639–653 (2018). https://doi.org/10.1007/s10126-018-9835-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9835-3