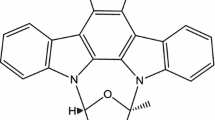
Abstract
Neurodegenerative disorders such as Alzheimer’s disease (AD), Glioblastoma multiforme (GBM), Amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD) are some of the most prevalent neurodegenerative disorders in humans. Even after a variety of advanced therapies, prognosis of all these disorders is not favorable, with survival rates of 14–20 months only. To further improve the prognosis of these disorders, it is imperative to discover new compounds which will target effector proteins involved in these disorders. In this study, we have focused on in silico screening of marine compounds against multiple target proteins involved in AD, GBM, ALS, and PD. Fifty marine-origin compounds were selected from literature, out of which, thirty compounds passed ADMET parameters. Ligand docking was performed after ADMET analysis for AD, GBM, ALS, and PD-associated proteins in which four protein targets Keap1, Ephrin A2, JAK3 Kinase domain, and METTL3-METTL14 N6-methyladenosine methyltransferase (MTA70) were found to be binding strongly with the screened compound Dioxinodehydroeckol (DHE). Molecular dynamics simulations were performed at 100 ns with triplicate runs to validate the docking score and assess the dynamics of DHE interactions with each target protein. The results indicated Dioxinodehydroeckol, a novel marine compound, to be a putative inhibitor among all the screened molecules, which might be effective against multiple target proteins involved in neurological disorders, requiring further in vitro and in vivo validations.








Similar content being viewed by others
Data Availability
The authors state that the data generated to reach the conclusion of this article are included in the article.
Abbreviations
- Aβ:
-
Amyloid beta
- Ach:
-
Acetylcholine
- AD:
-
Alzheimer’s Disease
- ALS:
-
Amyotrophic lateral sclerosis
- APOE4:
-
Apolipoprotein E4
- APP:
-
Amyloid precursor protein
- BBB:
-
Blood: Brain Barrier
- CNS:
-
Central Nervous System
- CADD:
-
Computer: aided drug design
- DHE:
-
Dioxinodehydroeckol
- GBM:
-
Glioblastoma multiforme
- GO:
-
Gene Ontology
- LRRK2:
-
Leucine-rich repeat kinase 2
- NMDA:
-
N-methyl D-aspartate
- NIST:
-
National Institute of Standards and Technology
- PD:
-
Parkinson’s Disease
- RMSD:
-
Root Mean Square Deviation
- RMSF:
-
Root mean square fluctuation
- SSE:
-
Secondary structural elements
- TMZ:
-
Temozolomide
- TYK2:
-
Tyrosine kinase 2
- VEGFR:
-
Vascular endothelial growth factor receptor
- WHO:
-
World Health Organization
- ZFD:
-
Zinc Finger Domain
References
Tarawneh, R., & Holtzman, D. M. (2012). The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harbor Perspectives in Medicine, 2(5), a006148.
Breijyeh, Z., & Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules, 25(24), 5789.
Kim, N., & Lee, H. J. (2020). Target enzymes considered for the treatment of Alzheimer’s disease and parkinson’s disease. BioMedical Research International, 2020, 2010728.
Vitovcova, B., Skarkova, V., Rudolf, K., & Rudolf, E. (2020). Biology of glioblastoma multiforme-exploration of mitotic catastrophe as a potential treatment modality. International Journal Molecular Science, 21(15), 5324.
Silantyev, A. S., Falzone, L., Libra, M., Gurina, O. I., Kardashova, K. S., Nikolouzakis, T. K., Nosyrev, A. E., Sutton, C. W., Mitsias, P. D., & Tsatsakis, A. (2019). Current and future trends on diagnosis and prognosis of glioblastoma: From molecular biology to proteomics. Cells, 8(8), 863.
Ou, A., Yung, W. K. A., & Majd, N. (2020). Molecular mechanisms of treatment resistance in glioblastoma. International Journal Molecular Science, 22(1), 351.
Drake, L. R., Hillmer, A. T., & Cai, Z. (2020). Approaches to PET imaging of glioblastoma. Molecules, 25(3), 568.
van Tellingen, O., Yetkin-Arik, B., de Gooijer, M. C., Wesseling, P., Wurdinger, T., & de Vries, H. E. (2015). Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resistance Updates, 19, 1–12.
Abou-Khalil, B. (2008). Levetiracetam in the treatment of epilepsy. Neuropsychiatric Disease and Treatment, 4(3), 507–523.
Batra, G., Jain, M., Singh, R. S., Sharma, A. R., Singh, A., Prakash, A., & Medhi, B. (2019). Novel therapeutic targets for amyotrophic lateral sclerosis. Indian Journal Pharmacol, 51(6), 418–425.
Oggiano, R., Pisano, A., Sabalic, A., Farace, C., Fenu, G., Lintas, S., Forte, G., Bocca, B., & Madeddu, R. (2021). An overview on amyotrophic lateral sclerosis and cadmium. Neurological Sciences, 42(2), 531–537.
Nowicka, N., Juranek, J., Juranek, J. K., & Wojtkiewicz, J. (2019). Risk factors and emerging therapies in amyotrophic lateral sclerosis. International Journal Molecular Science, 20(11), 2616.
Wakade, C., & Chong, R. (2014). A novel treatment target for Parkinson’s disease. Journal of the Neurological Sciences, 347(1–2), 34–38.
Simon, D. K., Tanner, C. M., & Brundin, P. (2020). Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clinics in Geriatric Medicine, 36(1), 1–12.
Dexter, D. T., & Jenner, P. (2013). Parkinson disease: From pathology to molecular disease mechanisms. Free Radical Biology & Medicine, 62, 132–144.
Teng, Y., Liu, Z., Chen, X., Liu, Y., Geng, F., Le, W., Jiang, H., & Yang, L. (2021). Conditional deficiency of m6A methyltransferase Mettl14 in substantia nigra alters dopaminergic neuron function. Journal of Cellular and Molecular Medicine, 25(17), 8567–8572.
Cacabelos, R. (2017). Parkinson’s disease: from pathogenesis to pharmacogenomics. International Journal of Molecular Sciences, 18(3), 551.
Krüger, A., Maltarollo, V. G. , Wrenger, C., & Kronenberger, T. (2019). ADME Profiling in Drug Discovery and a New Path Paved on Silica. In V. Gaitonde, P. Karmakar, & A. Trivedi (Eds.), Drug Discovery and Development - New Advances. IntechOpen.
Lu, S. H., Wu, J. W., Liu, H. L., Zhao, J. H., Liu, K. T., Chuang, C. K., Lin, H. Y., Tsai, W. B., & Ho, Y. (2011). The discovery of potential acetylcholinesterase inhibitors: A combination of pharmacophore modeling, virtual screening, and molecular docking studies. Journal of Biomedical Science, 18, 8.
Ferreira, L. G., Dos Santos, R. N., Oliva, G., & Andricopulo, A. D. (2015). Molecular docking and structure-based drug design strategies. Molecules, 20(7), 13384–13421.
Meng, X. Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: A powerful approach for structure-based drug discovery. Current Computer-Aided Drug Design, 7(2), 146–157.
Sana Khalid, M. A., Saeed, F., Bader-Ul-Ain, H., & Suleria, H. A. R. (2018). Therapeutic Potential of Seaweed Bioactive Compounds, in Seaweed Biomaterials. Intechopen.
Huang, R., Zhou, X., Xu, T., Yang, X., & Liu, Y. (2010). Diketopiperazines from marine organisms. Chemistry & Biodiversity, 7(12), 2809–2829.
Varijakzhan, D., Loh, J. Y., Yap, W. S., Yusoff, K., Seboussi, R., Lim, S. E., Lai, K. S., & Chong, C. M. (2021). Bioactive compounds from marine sponges: Fundamentals and applications. Marine Drugs, 19(5), 246.
Hamed, I., Özogul, F., Özogul, Y., & Regenstein, J. M. (2015). Marine bioactive compounds and their health benefits: A review. Comprehensive Reviews in Food Science and Food Safety, 14, 446–465.
Yan, J., Liu, W., Cai, J., Wang, Y., Li, D., Hua, H., & Cao, H. (2021). Advances in phenazines over the past decade: Review of their pharmacological activities, mechanisms of action, biosynthetic pathways and synthetic strategies. Marine Drugs, 19(11), 610.
Mehbub, M. F., Lei, J., Franco, C., & Zhang, W. (2014). Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Marine Drugs, 12(8), 4539–4577.
Donia, M., & Hamann, M. T. (2003). Marine natural products and their potential applications as anti-infective agents. The Lancet Infectious Diseases, 3(6), 338–348.
Lever, J., Brkljaca, R., Kraft, G., & Urban, S. (2020). Natural products of marine macroalgae from South Eastern Australia, with emphasis on the Port Phillip Bay and heads regions of Victoria. Marine Drugs, 18(3), 142.
Hur, S., Lee, H., Kim, Y., Lee, B. H., Shin, J., & Kim, T. Y. (2008). Sargaquinoic acid and sargachromenol, extracts of sargassum sagamianum, induce apoptosis in HaCaT cells and mice skin: Its potentiation of UVB-induced apoptosis. European Journal of Pharmacology, 582(1–3), 1–11.
Cotas, J., Leandro, A., Monteiro, P., Pacheco, D., Figueirinha, A., Goncalves, A. M. M., da Silva, G. J., & Pereira, L. (2020). Seaweed phenolics: From extraction to applications. Marine Drugs, 18(8), 384.
Lakshmi, S., Prakash, P., Essa, M. M., Qoronfleh, W. M., Akbar, M., Song, B. J., Kumar, S., & Elumalai, P. (2018). Marine derived bioactive compounds for treatment of Alzheimer’s disease. Frontiers in Bioscience (Elite Edition), 10(3), 537–548.
Abdallah, M. M., Fernandez, N., Matias, A. A., & Bronze, M. D. R. (2020). Hyaluronic acid and chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydrate Polymers, 243, 116441.
Silva, M., Seijas, P., & Otero, P. (2021). Exploitation of marine molecules to manage Alzheimer’s disease. Marine Drugs, 19(7), 373.
Adnan, M., Alshammari, E., Patel, M., Amir Ashraf, S., Khan, S., & Hadi, S. (2018). Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: Necessity for green chemistry. PeerJ, 6, e5049.
Pires, D. E., Blundell, T. L., & Ascher, D. B. (2015). pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of Medicinal Chemistry, 58(9), 4066–4072.
Sudom, A., Talreja, S., Danao, J., Bragg, E., Kegel, R., Min, X., Richardson, J., Zhang, Z., Sharkov, N., Marcora, E., Thibault, S., Bradley, J., Wood, S., Lim, A. C., Chen, H., Wang, S., Foltz, I. N., Sambashivan, S., & Wang, Z. (2018). Molecular basis for the loss-of-function effects of the Alzheimer’s disease-associated R47H variant of the immune receptor TREM2. Journal of Biological Chemistry, 293(32), 12634–12646.
Lo, S. C., Li, X., Henzl, M. T., Beamer, L. J., & Hannink, M. (2006). Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO Journal, 25(15), 3605–3617.
Hong, L., Koelsch, G., Lin, X., Wu, S., Terzyan, S., Ghosh, A. K., Zhang, X. C., & Tang, J. (2000). Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science, 290(5489), 150–153.
Himanen, J. P., Goldgur, Y., Miao, H., Myshkin, E., Guo, H., Buck, M., Nguyen, M., Rajashankar, K. R., Wang, B., & Nikolov, D. B. (2009). Ligand recognition by A-class Eph receptors: Crystal structures of the EphA2 ligand-binding domain and the EphA2/ephrin-A1 complex. EMBO Reports, 10(7), 722–728.
Zhang, X., Pickin, K. A., Bose, R., Jura, N., Cole, P. A., & Kuriyan, J. (2007). Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature, 450(7170), 741–744.
Chen, Y. W., Allen, M. D., Veprintsev, D. B., Lowe, J., & Bycroft, M. (2004). The structure of the AXH domain of spinocerebellar ataxin-1. Journal of Biological Chemistry, 279(5), 3758–3765.
Duan, J. J., Lu, Z., Jiang, B., Yang, B. V., Doweyko, L. M., Nirschl, D. S., Haque, L. E., Lin, S., Brown, G., Hynes, J., Jr., Tokarski, J. S., Sack, J. S., Khan, J., Lippy, J. S., Zhang, R. F., Pitt, S., Shen, G., Pitts, W. J., Carter, P. H., … Wrobleski, S. T. (2014). Discovery of pyrrolo[1,2-b]pyridazine-3-carboxamides as Janus kinase (JAK) inhibitors. Bioorganic & Medicinal Chemistry Letters, 24(24), 5721–5726.
Briknarová, K., Grishaev, A., Bányai, L., Tordai, H., Patthy, L., & Llinás, M. (1999). The second type II module from human matrix metalloproteinase 2: Structure, function and dynamics. Structure, 7(10), 1235-S2.
Cavalier, M. C., Kim, S. G., Neau, D., & Lee, Y. H. (2012). Molecular basis of the fructose-2,6-bisphosphatase reaction of PFKFB3: Transition state and the C-terminal function. Proteins, 80(4), 1143–53.
Okatsu, K., Sato, Y., Yamano, K., Matsuda, N., Negishi, L., Takahashi, A., Yamagata, A., Goto-Ito, S., Mishima, M., Ito, Y., Oka, T., Tanaka, K., & Fukai, S. (2018). Structural insights into ubiquitin phosphorylation by PINK1. Science and Reports, 8(1), 10382.
Gilsbach, B. K., Messias, A. C., Ito, G., Sattler, M., Alessi, D. R., Wittinghofer, A., & Kortholt, A. (2015). Structural characterization of LRRK2 inhibitors. Journal of Medicinal Chemistry, 58(9), 3751–6.
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., & Ferrin, T. E. (2004). UCSF Chimera–a visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25(13), 1605–12.
Ropp, P. J., Spiegel, J. O., Walker, J. L., Green, H., Morales, G. A., Milliken, K. A., Ringe, J. J., & Durrant, J. D. (2019). Gypsum-DL: An open-source program for preparing small-molecule libraries for structure-based virtual screening. Journal Cheminformation, 11(1), 34.
Bowers, K.J.a.C., David E. and Xu, Huafeng and Dror, Ron O. and Eastwood, Michael P. and Gregersen, Brent A. and Klepeis, John L. and Kolossvary, Istvan and Moraes, Mark A. and Sacerdoti, Federico D. and Salmon, John K. and Shan, Yibing and Shaw, David E. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. in SC '06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing. . 2006. IEEE.
Hildebrand, P. W., Rose, A. S., & Tiemann, J. K. S. (2019). Bringing Molecular dynamics simulation data into view. Trends in Biochemical Sciences, 44(11), 902–913.
Rasheed, M. A., Iqbal, M. N., Saddick, S., Ali, I., Khan, F. S., Kanwal, S., Ahmed, D., Ibrahim, M., Afzal, U., & Awais, M. (2021). Identification of lead compounds against Scm (fms10) in enterococcus faecium using computer aided drug designing. Life (Basel), 11(2), 77.
Shivakumar, D., Williams, J., Wu, Y., Damm, W., Shelley, J., & Sherman, W. (2010). Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. Journal of Chemical Theory and Computation, 6(5), 1509–19.
Maiorov, V. N., & Crippen, G. M. (1994). Significance of root-mean-square deviation in comparing three-dimensional structures of globular proteins. Journal of Molecular Biology, 235(2), 625–34.
Ramsey, C. P., Glass, C. A., Montgomery, M. B., Lindl, K. A., Ritson, G. P., Chia, L. A., Hamilton, R. L., Chu, C. T., & Jordan-Sciutto, K. L. (2007). Expression of Nrf2 in neurodegenerative diseases. Journal of Neuropathology and Experimental Neurology, 66(1), 75–85.
Sarlette, A., Krampfl, K., Grothe, C., Neuhoff, N., Dengler, R., & Petri, S. (2008). Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology, 67(11), 1055–62.
Kerr, F., Sofola-Adesakin, O., Ivanov, D. K., Gatliff, J., Gomez Perez-Nievas, B., Bertrand, H. C., Martinez, P., Callard, R., Snoeren, I., Cocheme, H. M., Adcott, J., Khericha, M., Castillo-Quan, J. I., Wells, G., Noble, W., Thornton, J., & Partridge, L. (2017). Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genetics, 13(3), e1006593.
Horie, Y., Suzuki, T., Inoue, J., Iso, T., Wells, G., Moore, T. W., Mizushima, T., Dinkova-Kostova, A. T., Kasai, T., Kamei, T., Koshiba, S., & Yamamoto, M. (2021). Molecular basis for the disruption of Keap1-Nrf2 interaction via hinge & latch mechanism. Communications Biology, 4(1), 576.
Guo, W., Vandoorne, T., Steyaert, J., Staats, K. A., & Van Den Bosch, L. (2020). The multifaceted role of kinases in amyotrophic lateral sclerosis: Genetic, pathological and therapeutic implications. Brain, 143(6), 1651–1673.
Trieu, V. N., Liu, R., Liu, X. P., & Uckun, F. M. (2000). A specific inhibitor of janus kinase-3 increases survival in a transgenic mouse model of amyotrophic lateral sclerosis. Biochemical and Biophysical Research Communications, 267(1), 22–5.
Tan, L., Akahane, K., McNally, R., Reyskens, K. M. S. E., Ficarro, S. B., Liu, S., Herter-Sprie, G. S., Koyama, S., Pattison, M. J., Labella, K., Johannessen, L., Akbay, E. A., Wong, K.-K., Frank, D. A., Marto, J. A., Look, T. A., Arthur, J. S. C., Eck, M. J., & Gray, N. S. (2015). Development of selective covalent janus kinase 3 inhibitors. Journal of Medicinal Chemistry, 58(16), 6589–6606.
Casimiro-Garcia, A., Trujillo, J. I., Vajdos, F., Juba, B., Banker, M. E., Aulabaugh, A., Balbo, P., Bauman, J., Chrencik, J., Coe, J. W., Czerwinski, R., Dowty, M., Knafels, J. D., Kwon, S., Leung, L., Liang, S., Robinson, R. P., Telliez, J.-B., Unwalla, R., … Thorarensen, A. (2018). Identification of cyanamide-based janus kinase 3 (JAK3) covalent inhibitors. Journal of Medicinal Chemistry, 61(23), 10665–10699.
Forster, M., Chaikuad, A., Dimitrov, T., Doring, E., Holstein, J., Berger, B. T., Gehringer, M., Ghoreschi, K., Muller, S., Knapp, S., & Laufer, S. A. (2018). Development, optimization, and structure-activity relationships of covalent-reversible JAK3 inhibitors based on a tricyclic Imidazo[5,4- d]pyrrolo[2,3- b]pyridine scaffold. Journal of Medicinal Chemistry, 61(12), 5350–5366.
Telliez, J.-B., Dowty, M. E., Wang, L., Jussif, J., Lin, T., Li, L., Moy, E., Balbo, P., Li, W., Zhao, Y., Crouse, K., Dickinson, C., Symanowicz, P., Hegen, M., Banker, M. E., Vincent, F., Unwalla, R., Liang, S., Gilbert, A. M., … Thorarensen, A. (2016). Discovery of a JAK3-selective inhibitor: Functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chemical Biology, 11(12), 3442–3451.
Thorarensen, A., Dowty, M. E., Banker, M. E., Juba, B., Jussif, J., Lin, T., Vincent, F., Czerwinski, R. M., Casimiro-Garcia, A., Unwalla, R., Trujillo, J. I., Liang, S., Balbo, P., Che, Y., Gilbert, A. M., Brown, M. F., Hayward, M., Montgomery, J., Leung, L., … Telliez, J. B. (2017). Design of a janus kinase 3 (JAK3) specific inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop -2-en-1-one (PF-06651600) allowing for the interrogation of JAK3 signaling in humans. Journal of Medicinal Chemistry, 60(5), 1971–1993.
Xiao, T., Xiao, Y., Wang, W., Tang, Y. Y., Xiao, Z., & Su, M. (2020). Targeting EphA2 in cancer. Journal of Hematology & Oncology, 13(1), 114.
Singh, D. R., Kanvinde, P., King, C., Pasquale, E. B., & Hristova, K. (2018). The EphA2 receptor is activated through induction of distinct, ligand-dependent oligomeric structures. Communication Biology, 1, 15.
Schapira, A. H., & Jenner, P. (2011). Etiology and pathogenesis of Parkinson’s disease. Movement Disorders, 26(6), 1049–55.
Jiang, L., Li, X., Wang, S., Yuan, Z., & Cheng, J. (2022). The role and regulatory mechanism of m(6)A methylation in the nervous system. Frontiers in Genetics, 13, 962774.
You, S., Su, X., Ying, J., Li, S., Qu, Y., & Mu, D. (2022). Research progress on the role of RNA m6A modification in glial cells in the regulation of neurological diseases. Biomolecules, 12(8), 1158.
Lei, C., & Wang, Q. (2022). The progression of N6-methyladenosine study and its role in neuropsychiatric disorders. International Journal of Molecular Sciences, 23(11), 5922.
Han, M., Liu, Z., Xu, Y., Liu, X., Wang, D., Li, F., Wang, Y., & Bi, J. (2020). Abnormality of m6A mRNA methylation is involved in Alzheimer’s disease. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2020.00098
Kong, C. S., Kim, J. A., Yoon, N. Y., & Kim, S. K. (2009). Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food and Chemical Toxicology, 47(7), 1653–8.
Moon, H. E., Islam, N., Ahn, B. R., Chowdhury, S. S., Sohn, H. S., Jung, H. A., & Choi, J. S. (2011). Protein tyrosine phosphatase 1B and alpha-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Bioscience, Biotechnology, and Biochemistry, 75(8), 1472–80.
Ryu, B., Ahn, B. N., Kang, K. H., Kim, Y. S., Li, Y. X., Kong, C. S., Kim, S. K., & Kim, D. G. (2015). Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. Journal of Photochemistry and Photobiology B: Biology, 153, 352–7.
Kim, S. K., & Kong, C. S. (2010). Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chemico-Biological Interactions, 186(1), 24–9.
Ahn, B. N., Karadeniz, F., Kong, C. S., Nam, K. H., Jang, M. S., Seo, Y., & Kim, H. S. (2016). Dioxinodehydroeckol enhances the differentiation of osteoblasts by regulating the expression of phospho-smad1/5/8. Marine Drugs, 14(9), 168.
Lee, M. S., Yoon, H. D., Kim, J. I., Choi, J. S., Byun, D. S., & Kim, H. R. (2012). Dioxinodehydroeckol inhibits melanin synthesis through PI3K/Akt signalling pathway in alpha-melanocyte-stimulating hormone-treated B16F10 cells. Experimental Dermatology, 21(6), 471–3.
Bak, S. S., Ahn, B. N., Kim, J. A., Shin, S. H., Kim, J. C., Kim, M. K., Sung, Y. K., & Kim, S. K. (2013). Ecklonia cava promotes hair growth. Clinical and Experimental Dermatology, 38(8), 904–10.
Eom, S. H., Lee, S. H., Yoon, N. Y., Jung, W. K., Jeon, Y. J., Kim, S. K., Lee, M. S., & Kim, Y. M. (2012). alpha-Glucosidase- and alpha-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. Journal of the Science of Food and Agriculture, 92(10), 2084–90.
Acknowledgements
The authors would like to acknowledge Mr. Muhammad Nasir Iqbal for providing the simulation facility and Mr. Nobendu Mukherjee for analyzing the MD results and assisting in article formatting.
Funding
Not received.
Author information
Authors and Affiliations
Contributions
FA and GS helped in writing the manuscript and idea generation; PS, BS, HS, and ST helped in writing the manuscript; MMR, MZA, HMB, and MK helped in revision and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors proclaim no competing interests.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, F., Sachdeva, P., Sachdeva, B. et al. Dioxinodehydroeckol: A Potential Neuroprotective Marine Compound Identified by In Silico Screening for the Treatment and Management of Multiple Brain Disorders. Mol Biotechnol 66, 663–686 (2024). https://doi.org/10.1007/s12033-022-00629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00629-3