Abstract
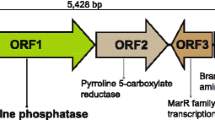
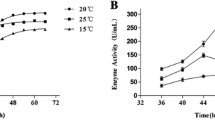
The psychrophilic marine bacterium, Cobetia marina, recovered from the mantle tissue of the marine mussel, Crenomytilus grayanus, which contained a gene encoding alkaline phosphatase (AP) with apparent biotechnology advantages. The enzyme was found to be more efficient than its counterparts and showed k cat value 10- to 100-fold higher than those of all known commercial APs. The enzyme did not require the presence of exogenous divalent cations and dimeric state of its molecule for activity. The recombinant enzyme (CmAP) production and purification were optimized with a final recovery of 2 mg of the homogenous protein from 1 L of the transgenic Escherichia coli Rosetta(DE3)/Pho40 cells culture. CmAP displayed a half-life of 16 min at 45 °C and 27 min at 40 °C in the presence of 2 mM EDTA, thus suggesting its relative thermostability in comparison with the known cold-adapted analogues. A high concentration of EDTA in the incubation mixture did not appreciably inhibit CmAP. The enzyme was stable in a wide range of pH (6.0–11.0). CmAP exhibited its highest activity at the reaction temperature of 40–50 °C and pH 9.5–10.3. The structural features of CmAP could be the reason for the increase in its stability and catalytic turnover. We have modeled the CmAP 3D structure on the base of the high-quality experimental structure of the close homologue Vibrio sp. AP (VAP) and mutated essential residues predicted to break Mg2+ bonds in CmAP. It seems probable that the intrinsically tight binding of catalytic and structural metal ions together with the flexibility of intermolecular and intramolecular links in CmAP could be attributed to the adapted mutualistic lifestyle in oceanic waters.












Similar content being viewed by others
References
Ammerman JW, Azam F (1985) Bacterial 5’-nucleotidase in aquatic ecosystems: a novel mechanism of phosphorus regeneration. Science 227:1338–1340
Aono T, Maldonado-Mendoza IE, Dewbre GR, Harrison MJ, Saito M (2004) Expression of alkaline phosphatase genes in arbuscular mycorrhizas. New Phytol 162:525–534
Asgeirsson B, Nielsen BN, Hojrup P (2003) Amino acid sequence of the cold-active alkaline phosphatase from Atlantic cod (Gadus morhua). Comp Biochem Physiol B Biochem Mol Biol 136:45–60
Babor M, Greenblatt HM, Edelman M, Sobolev V (2005) Flexibility of metal binding sites in proteins on a database scale. Proteins 59:221–230
Bihani SC, Das A, Nilgiriwala KS, Prashar V, Pirocchi M, Apte SK, Ferrer JL, Hosur MV (2011) X-Ray structure reveals a new class and provides insight into evolution of alkaline phosphatases. PLoS One 6:e22767
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein-dye binding. Anal Biochem 72:248–254
De Backer M, McSweeney S, Rasmussen HB, Riise BW, Lindley P, Hough E (2002) The 1.9 A crystal structure of heat-labile shrimp alkaline phosphatase. J Mol Biol 318:1265–1274
Dell’Anno A, Danavaro R (2005) Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science 309:2179
Dorozhkin SV (2009) Calcium orthophosphates in nature, biology and medicine. Materials 2:399–498
Gudjónsdóttir K, Asgeirsson B (2008) Effects of replacing active site residues in a cold-active alkaline phosphatase with those found in its mesophilic counterpart from Escherichia coli. FEBS J 275:117–127
Hauksson BJ, Andresson OS, Asgeirsson B (2000) Heat-labile bacterial alkaline phosphatase from a marine Vibrio sp. Enzyme Microb Technol 27:66–73
Helland R, Larsen RL, Asgeirsson B (2009) The 1.4 A crystal structure of the large and cold-active Vibrio sp. alkaline phosphatase. Biochim Biophys Acta 1794:297–308
Henney NC, Li B, Elford C, Reviriego P, Campbell AK, Wann KT (2009) A large-conductance (BK) potassium channel subtype affects both growth and mineralization of human osteoblasts. Am J Physiol Cell Physiol 297:1397–1408
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comp 4:435–447
Hoppe HG (2003) Phosphatase activity in the sea. Hydrobiologia 493:187–200
Hulett FM, Kim EE, Bookstein C, Kapp NV, Edwards CW, Wyckoff HW (1991) Bacillus subtilis alkaline phosphatases III and IV. Cloning, sequencing, and comparisons of deduced amino acid sequence with Escherichia coli alkaline phosphatase three-dimensional structure. J Biol Chem 266:1077–1084
Ishibashi M, Yamashita S, Tokunaga M (2005) Characterization of halophilic alkaline phosphatase from Halomonas sp. 593, a moderately halophilic bacterium. Biosci Biotechnol Biochem 69:1213–1216
Ivanova EP, Christen R, Sawabe T, Alexeeva YV, Lysenko AM, Chelomin VP, Mikhailov VV (2005) Presence of ecophysiologically diverse populations within cobetia marina strains isolated from marine invertabrate, algae and the environments. Microb Environ 20:200–207
Janeway CM, Xu X, Murphy JE, Chaidaroglou A, Kantrowitz ER (1993) Magnesium in the active site of Escherichia coli alkaline phosphatase is important for both structural stabilization and catalysis. Biochemistry 32:1601–1609
Kim JW, Peterson T, Bee G, Hulett FM (1998) Bacillus licheniformis MC14 alkaline phosphatase I gene with an extended COOH-terminus. FEMS Microbiol Lett 159:47–58
Kobori H, Sullivan CW, Shizya H (1984) Heat-labile alkaline phosphatase from Antarctic bacteria: rapid 5’-end-labeling of nucleic acids. Proc Natl Acad Sci U S A 81:6691–6695
Kozlenkov A, Manes T, Hoylaerts MF, Millan JL (2002) Function assignment to conserved residues in mammalian alkaline phosphatases. J Biol Chem 277:22992–22999
Laemmli UK (1970) Cleavage of structural proteins during the assembly of thehead of the bacteriophage T7. Nature 227:80–685
Le Du MH, Stigbrand T, Taussig MJ, Menez A, Stura EA (2001) Crystal structure of alkaline phosphatase from human placenta at 1.8 A resolution. Implication for a substrate specificity. J Biol Chem 276:9158–9165
Leveque I, Cusack M, Davis SA, Mann S (2004) Promotion of fluorapatite crystallization by soluble-matrix proteins from Lingula Anatina shells. Angew Chem Int Ed Engl 43:885–888
Li H, Robertson AD, Jensen JH (2005) Very fast emperical prediction and interpretation of protein pKa values. Proteins 61:704–721
McComb RB, Bowers GN, Posen S (1979) Alkaline phosphatase. Plenum Press, NY
Millan JL (2006) (2006) Alkaline phosphatases structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purin Sign 2:335–341
Moult J (2007) Comparative modeling in structural genomics. Structure 16:14–16
Murakawa T, Yamagata H, Tsuruta H, Aizono Y (2002) Cloning of cold-active alkaline phosphatase gene of a psychrophile, Shewanella sp., and expression of the recombinant enzyme. Biosci Biotechnol Biochem 66:754–761
Nasu E, Ichiyanagi A, Gomi K (2012) Cloning and expression of a highly active recombinant alkaline phosphatase from psychrotrophic Cobetia marina. Biotechnol Lett 34:321–8
Olsson MHM, Søndergard SR, Rostkowski M, Jensen JH (2011) PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theory Comp 7:525–537
Paerl HW, Merkel SM (1982) Differential phosphorus assimilation in attached vs. unattached microorganisms. Arch Hydrobiol 93:125–134
Page MJ, Di Sera E (2006) Role of Na+ and K+ in enzyme function. Physiol Rev 86:1049–1092
Plisova EY, Balabanova LA, Ivanova EP, Kozhemyako VB, Mikhailov VV, Agafonova EV, Rasskazov VA (2005) A highly active alkaline phosphatase from the marine bacterium Cobetia. Mar Biotechnol 7:173–178
Poltorak OM, Chukhray ES, Torshin IY, Atyaksheva LF, Trevan MD, Chaplin MF (1999) Catalytic properties, stability and the structure of the conformational lock in the alkaline phosphatase from Escherichia coli. J Mol Catalys B 7:165–172
Qian B, Raman S, Das R, Bradley P, McCoy AJ, Read RJ, Baker D (2007) High-resolution structure prediction and the crystallographic phase problem. Nature 450:259–261
Rahmanov S, Kulakovskiy I, Uroshlev L, Makeev V (2010) Empirical potentials for ion binding in proteins. J Bioinform Comput Biol 8:427–435
Rina M, Pozidis C, Mavromatis K, Tzanodaskalaki M, Kokkinidis M, Bouriotis V (2000) Alkaline phosphatase from the Antarctic strain TAB5. Eur J Biochem 267:1230–1238
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Suzuki Y, Mizutani Y, Tsuji T, Ohtani N, Takano K, Haruki M, Morikawa M, Kanaya S (2005) Gene cloning, overproduction, and characterization of thermolabile alkaline phosphatase from a psychrotrophic bacterium. Biosci Biotechnol Biochem 69:364–373
Van der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible and free. J Comp Chem 26:1701–1718
Van der Spoel D, Lindahl E, Hess B, and the GROMACS development team, GROMACS User Manual version 4.6.3 (2013), www.gromacs.org
Wang E, Koutsioulis D, Leiros HK, Andersen OA, Bouriotis V, Hough E, Heikinheimo P (2007) Crystal structure of alkaline phosphatase from the antarctic bacterium TAB5. J Mol Biol 366:1318–1331
Zaheer R, Morton R, Proudfoot M, Yakunin A, Finan TM (2009) Genetic and biochemical properties of an alkaline phosphatase PhoX family protein found in many bacteria. Environ Microbiol 11:1572–87
Zalatan JG, Fenn TD, Herschlag D (2008) Comparative enzymology in the alkaline phosphatase superfamily to determine the catalytic role of an active-site metal ion. J Mol Biol 384:1174–1189
Zappa S, Rolland J-L, Flament D, Gueguen Y, Boudrant J, Dietrich J (2001) Characterization of a highly thermostable alkaline phosphatase from the euryarchaeon Pyrococcus abyssi. Appl Environ Microbiol 67:4504–4511
Acknowledgments
The work was supported by grant from RFBR 12-04-00825-a and the projects 13 НТПI-12, 12-I-П6-10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golotin, V., Balabanova, L., Likhatskaya, G. et al. Recombinant Production and Characterization of a Highly Active Alkaline Phosphatase from Marine Bacterium Cobetia marina . Mar Biotechnol 17, 130–143 (2015). https://doi.org/10.1007/s10126-014-9601-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-014-9601-0