Abstract
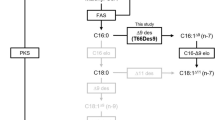
Thraustochytrids, unicellular eukaryotic marine protists, accumulate polyunsaturated fatty acids. Here, we report the molecular cloning and functional characterization of two fatty acid elongase genes (designated tselo1 and tselo2), which could be involved in the desaturase/elongase (standard) pathway in Thraustochytrium sp. ATCC 26185. TsELO1, the product of tselo1 and classified into a Δ6 elongase group by phylogenetic analysis, showed strong C18-Δ6 elongase activity and relatively weak C18-Δ9 and C20-Δ5 activities when expressed in the budding yeast Saccharomyces cerevisiae. TsELO2, classified into a Δ9 elongase subgroup, showed only C16-Δ9 activity. When expressed in Aurantiochytrium limacinum mh0186 using a thraustochytrid-derived promoter and a terminator, TsELO1 exhibited almost the same specificity as expressed in the yeast but TsELO2 showed weak C18-Δ9 activity, in addition to its main C16-Δ9 activity. These results suggest that TsELO1 functions not only as a C18-Δ6 and a C20-Δ5 elongase in the main route but also as a C18-Δ9 elongase in the alternative route of standard pathway, while TsELO2 functions mainly as a C16-Δ9 elongase generating vaccenic acid (C18:1n−7) in thraustochytrids. This is the first report describing a fatty acid elongase harboring C16-Δ9 activity in thraustochytrids.






Similar content being viewed by others
Abbreviations
- ALA:
-
α-Linolenic acid (C18:3n−3)
- ARA:
-
Arachidonic acid (C20:4n−6)
- DGLA:
-
Dihomo-γ-linolenic acid (C20:3n−6)
- DHA:
-
Docosahexaenoic acid (C22:6n−3)
- n−3DPA:
-
Docosapentaenoic acid (C22:5n−3)
- EDA:
-
Eicosadienoic acid (C20:2n−6)
- EPA:
-
Eicosapentaenoic acid (C20:5n−3)
- ETA:
-
Eicosatetraenoic acid (C20:4n−3)
- ETrA:
-
Eicosatrienoic acid (C20:3n−3)
- FAME(s):
-
Fatty acid methyl ester(s)
- GLA:
-
γ-Linolenic acid (C18:3n−6)
- LA:
-
Linoleic acid (C18:2n−6)
- MUFA:
-
Monounsaturated fatty acid
- OA:
-
Oleic acid (C18:1n−9)
- PA:
-
Palmitoleic acid (C16:1n−7)
- PUFA(s):
-
Polyunsaturated fatty acid(s)
- SFA:
-
Saturated fatty acid
- STA:
-
Stearidonic acid (C18:4n−3)
- VA:
-
Vaccenic acid (C18:1n−7)
References
Allen EE, Bartlett DH (2002) Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology 148:1903–1913
Bannni S, Carta G, Angioni E, Murru E, Scanu P, Melis MP, Bauman DE, Fischer SM, Ip C (2001) Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver. J Lipid Res 42:1056–1061
Chen D-C, Yang B-C, Kuo T-T (1992) One-step transformation of yeast in stationary phase. Curr Genet 21:83–84
Fan KW, Aki T, Chen F, Jiang Y (2010) Enhanced production of squalene in the thraustochytrid Aurantiochytrium mangrovei by medium optimization and treatment with terbinafine. World J Microbiol Biotechnol 26:1303–1309
Gebauer SK, Chardigny J-M, Jakobsen MU, Lamarche B, Lock AL, Proctor SD, Baer DJ (2011) Effects of ruminant trans fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv Nutr 2:332–354
Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ (2008) Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 197:12–24
Hashimoto K, Yoshizawa AC, Okuda S, Kuma K, Goto S, Kanehisa M (2008) The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes. J Lipid Res 49:183–191
Jacome-Sosa MM, Lu J, Wang Y, Ruth MR, Wright DC, Reaney MJ, Shen J, Field CJ, Vine DF, Proctor SD (2010) Increased hypolipidemic benefits of cis-9, trans-11 conjugated linoleic acid in combination with trans-11 vaccenic acid in a rodent model of the metabolic syndrome, the JCR:LA-cp rat. Nutr Metab 7:60. doi:10.1186/1743-7075-7-60
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45:237–249
Jeh E-J, Kumaran RS, Hur B-K (2008) Lipid body formation by Thraustochytrium aureum (ATCC 34304) in response to cell age. Kor J Chem Eng 25:1103–1109
Jiang X, Qin L, Tian B, Shu Z, Huang J (2008) Cloning and expression of two elongase genes involved in the biosynthesis of docosahexaenoic acid in Thraustochytrium sp. FJN-10. Wei Sheng Wu Hsueh Pao 48:176–183
Kajikawa M, Yamato KT, Sakai Y, Fukuzawa H, Ohyama K, Kohchi T (2006) Isolation and functional characterization of fatty acid Δ5-elongase gene from the liverwort Marchantia polymorpha L. FEBS Lett 580:149–154
Kang D-H, Anbu P, Kim W-H, Hur B-K (2008) Coexpression of elo-like enzyme and Δ5, Δ4-desaturases derived from Thraustochytrium aureum ATCC 34304 and the production of DHA and DPA in Pichia pastoris. Biotechnol Bioproc Eng 13:483–490
Kaya K, Nakazawa A, Matsuura H, Honda D, Inouye I, Watanabe MM (2011) Thraustochytrid aurantiochytrium sp. 18W-13a accumulates high amounts of squalene. Biosci Biotechnol Biochem 75:2246–2248
Kobayashi T, Sakaguchi K, Matsuda T, Abe E, Hama Y, Hayashi M, Honda D, Okita Y, Sugimoto S, Okino N, Ito M (2011) Increase of eicosapentaenoic acid in thraustochytrids through thraustochytrid ubiquitin promoter-driven expression of a fatty acid Δ5 desaturase gene. Appl Environ Microbiol 77:3870–3876
Kumon Y, Kamisaka Y, Tomita N, Kimura K, Uemura H, Yokochi T, Yokoyama R, Honda D (2008) Isolation and characterization of a Δ5-desaturase from Oblongichytrium sp. Biosci Biotechnol Biochem 72:2224–2227
Lee J-C, Anbu P, Kim W-H, Noh M-J, Lee S-J, Seo J-W, Hur B-K (2008) Identification of Δ9-elongation activity from Thraustochytrium aureum by heterologous expression in Pichia pastoris. Biotechnol Bioproc Eng 13:524–532
Leonard AE, Pereira SL, Sprecher H, Huang YS (2004) Elongation of long-chain fatty acids. Prog Lipid Res 43:36–54
Li M, Ou X, Yang X, Guo D, Qian X, Xing L, Li M (2011) Isolation of a novel C18-Δ9 polyunsaturated fatty acid specific elongase gene from DHA-producing Isochrysis galbana H29 and its use for the reconstitution of the alternative Δ8 pathway in Saccharomyces cerevisiae. Biotechnol Lett 33:1823–1830
Lippmeier JC, Crawford KS, Owen CB, Rivas AA, Metz JG, Apt KE (2009) Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44:621–630
Matsuda T, Sakaguchi K, Hamaguchi R, Kobayashi T, Abe E, Hama Y, Hayashi M, Honda D, Okita Y, Sugimoto S, Okino N, Ito M (2012) The analysis of Δ12-fatty acid desaturase function revealed that two distinct pathways are active for the synthesis of polyunsaturated fatty acids in Thraustochytrium aureum ATCC34304. J Lipid Res 53:1210–1222
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by potyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Morita E, Kumon Y, Nakahara T, Kagiwada S, Noguchi T (2006) Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar Biotechnol 8:319–327
Nagano N, Sakaguchi K, Taoka Y, Okita Y, Honda D, Ito M, Hayashi M (2011) Detection of genes involved in fatty acid elongation and desaturation in thraustochytrid marine eukaryotes. J Oleo Sci 60:475–481
Qiu X, Hong H, MacKenzie SL (2001) Identification of a Δ4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexanoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J Biol Chem 276:31561–31566
Sakaguchi K, Matsuda M, Kobayashi T, Ohara J, Hamaguchi R, Abe E, Nagano N, Hayashi M, Ueda M, Honda D, Okita Y, Yaoka Y, Sugimoto S, Okino N, Ito M (2012) Versatile transformation system that is applicable to both multiple transgene expression and gene targeting for thraustochytrids. Appl Environ Microbiol 78:3193–3202
Salem NJ, Litman B, Kim H-Y, Gawrisch K (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36:945–959
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Taoka Y, Nagano N, Okita Y, Izumida H, Sugimoto S, Hayashi M (2009) Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp. strain mh0186. Mar Biotechnol 11:368–374
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4:12–21
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Viklund H, Elofsson A (2008) OCTOPUS: improving topology prediction by two-track ANN-based preference scores and an extended topological grammar. Bioinformatics 24:1662–1668
Wang Y, Lu J, Ruth MR, Goruk SD, Reaney MJ, Glimm DR, Vine DF, Field CJ, Proctor SD (2008) Trans-11 vaccenic acid dietary supplementation induces hypolipidemic effects in JCR:LA-cp rats. J Nutr 138:2117–2122
Yazawa K (1996) Production of eicosapentaenoic acid from marine bacteria. Lipids 31:S297–S300
Acknowledgments
We thank Dr. M. Hayashi at Miyazaki University for providing A. limacinum mh0186.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohara, J., Sakaguchi, K., Okita, Y. et al. Two Fatty Acid Elongases Possessing C18-Δ6/C18-Δ9/C20-Δ5 or C16-Δ9 Elongase Activity in Thraustochytrium sp. ATCC 26185. Mar Biotechnol 15, 476–486 (2013). https://doi.org/10.1007/s10126-013-9496-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-013-9496-1