Abstract
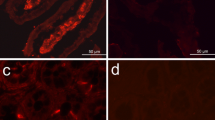
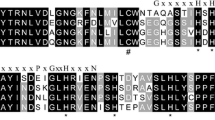
Sulfide is a natural, widely distributed, poisonous substance, and sulfide:quinone oxidoreductase (SQR) has been identified to be responsible for the initial oxidation of sulfide in mitochondria. In this study, full-length SQR cDNA was cloned from the echiuran worm Urechis unicinctus, a benthic organism living in marine sediments. The protein consisted of 451 amino acids with a theoretical pI of 8.98 and molecular weight of 50.5 kDa. Subsequently, the SQR mRNA expression in different tissues was assessed by real-time reverse transcription and polymerase chain reaction and showed that the highest expression was in midgut, followed by anal sacs and coelomic fluid cells, and then body wall and hindgut. Furthermore, activated SQR was obtained by dilution refolding of recombinant SQR expression in E. coli, and the refolded product showed optimal activity at 37 °C and pH 8.5 and K m for ubiquinone and sulfide at 15.6 µM and 40.3 µM, respectively. EDTA and GSH had an activating effect on refolded SQR, while Zn2+ caused decreased activity. Western blot showed that SQR in vivo was located in mitochondria and was ∼10 kDa heavier than the recombinant protein. In addition, SQR, detected by immunohistochemistry, was mainly located in the epithelium of all tissues examined. Ultrastructural observations of these tissues’ epithelium by transmission electron microscopy provided indirect cytological evidence for its mitochondrial location. Interesting aspects of the U. unicinctus SQR amino acid sequence, its catalytic mechanism, and the different roles of these tissues in sulfide metabolic adaptation are also discussed.










Similar content being viewed by others
References
Arp AJ, Menon JG, Julian D (1995) Multiple mechanisms provide tolerance to environmental sulfide in Urechis caupo. Integr Comp Biol 35:132–144
Bagarinao T (1992) Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat Toxicol 24:21–62
Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF, Rapp BA, Wheeler DL (1999) GenBank. Nucleic Acids Res 27:12–17
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Brito JA, Sousa FL, Stelter M, Bandeiras TM, Vonrhein C, Teixeira M, Pereira MM, Archer M (2009) Structural and functional insights into sulfide:quinone oxidoreductase. Biochemistry 48:5613–5622
Bronstein M, Schütz M, Hauska G, Padan E, Shahak Y (2000) Cyanobacterial sulfide-quinone reductase: cloning and heterologous expression. J Bacteriol 182:3336–3344
Carrico RJ, Blumberg WE, Peisach J (1978) The reversible binding of oxygen to sulfhemoglobin. J Biol Chem 253:7212–7215
Chen ZW, Jiang CY, She Q, Liu SJ, Zhou PJ (2005) Key role of cysteine residues in catalysis and subcellular localization of sulfur oxygenase-reductase of Acidianus tengchongensis. Appl Environ Microbiol 71:621–628
Dorner AJ, Bole DG, Kaufman RJ (1987) The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol 105:2665–2674
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Evans CL (1967) The toxicity of hydrogen sulphide and other sulphides. J Exp Physiol 52:231–248
Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RNF, Kristal BS, Brown AM (2002) Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem 277:10064–10072
Gieselmann V, Schmidt B, Von Figura K (1992) In vitro mutagenesis of potential N-glycosylation sites of arylsulfatase A. Effects on glycosylation, phosphorylation, and intracellular sorting. J Biol Chem 267:13262–13266
Griesbeck C, Hauska G, Schütz M (2000) Biological sulfide oxidation:sulfide-quinone reductase (SQR), the primary reaction. In: Pandalai SG (ed) Recent research developments in microbiology vol 4, rearch signpost. Trivadrum, India, pp 179–203
Griesbeck C, Schütz M, Schodl T, Bathe S, Nausch L, Mederer N, Vielreicher M, Hauska G (2002) Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry 41:11552–11565
Grieshaber MK, Völkel S (1998) Animal adaptations for tolerance and exploitation of poisonous sulfide. Annu Rev Physiol 60:33–53
Hance JM, Andrzejewski JE, Predmore BL, Dunlap KJ, Misiak KL, Julian D (2008) Cytotoxicity from sulfide exposure in a sulfide-tolerant marine invertebrate. J Exp Mar Biol 359:102–109
Hamby SE, Hirst JD (2008) Prediction of glycosylation sites using random forests. BMC Bioinformatics 9:500
Hildebrandt TM, Grieshaber MK (2008a) Redox regulation of mitochondrial sulfide oxidation in the lugworm, Arenicola marina. J Exp Biol 211:2617–2623
Hildebrandt TM, Grieshaber MK (2008b) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275:3352–3361
Jiménez-Castañoa L, Villamiel M, López-Fandiño R (2007) Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll 21:433–443
Joyner-Matos J, Predmore BL, Stein JR, Leeuwenburgh C, Julian D (2010) Hydrogen sulfide induces oxidative damage to RNA and DNA in a sulfide-tolerant marine invertebrate. Physiol Biochem Zool. doi:10.1086/597529 (in press)
Julian D, April KL, Patel S, Stein JR, Wohlgemuth SE (2005) Mitochondrial depolarization following hydrogen sulfide exposure in erythrocytes from a sulfide-tolerant marine invertebrate. J Exp Biol 208:4109–4122
Kraus D, Doeller J, Powell C (1996) Sulfide may directly modify cytoplasmic hemoglobin deoxygenation in Solemya reidi gills. J Exp Biol 199:1343–1352
Ma ZJ, Bao ZM, Kang KH, Yu L, Zhang ZF (2005) The changes of three components in coelomic fluid of Urechis unicinctus exposed to different concentrations of sulfide. Chin J Oceanol Limnol 23:104–109
Marcia M, Ermler U, Peng G, Michel H (2009) The structure of Aquifex aeolicus sulfide:quinone oxidoreductase, a basis to understand sulfide detoxification and respiration. Proc Natl Acad Sci U S A 106:9625–9630
Matzuk MM, Boime I (1988) The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol 106:1049–1059
Menon JG, Arp AJ (1992) Morphological adaptations of the respiratory hindgut of a marine echiuran worm. J Morphol 214:131–138
Menon JG, Arp AJ (1993) The integument of the marine echiuran worm Urechis caupo. J Morphol 185:440–454
Menon J, Arp AJ (1998) Ultrastructural evidence of detoxification in the alimentary canal of Urechis caupo. Integr Biol 117:307–317
Nicholls P (1975) The effect of sulphide on cytochrome aa3 isosteric and allosteric shifts of the reduced alpha-peak. Biochim Biophys Acta 396:24–35
Nicholls P, Kim JK (1982) Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Biochem Cell Biol 60:613–623
Olden K, Parent JB, White SL (1982) Carbohydrate moieties of glycoproteins a re-evaluation of their function. BBA-Rev Biomembranes 650:209–232
Powell MA, Arp AJ (1989) Hydrogen sulfide oxidation by abundant nonhemoglobin heme compounds in marine invertebrates from sulfide-rich habitats. J Exp Zool 249:121–132
Powell MA, Somero GN (1985) Sulfide oxidation occurs in the animal tissue of the gutless clam, Solemya reidi. Biol Bull 169:164–181
Powell MA, Somero GN (1986) Hydrogen sulfide oxidation is coupled to oxidative phosphorylation in mitochondria of Solemya reidi. Science 233:563–566
Scheibe R (1987) NADP+-malate dehydrogenase in C3-plants: regulation and role of a light-activated enzyme. Physiol Plant 71:393–400
Schroff G, Schöttler U (1977) Anaerobic reduction of fumarate in the body wall musculature of Arenicola marina (Polychaeta). J Comp Physiol B 116:325–336
Shibata H, Kobayashi S (2006) Characterization of a HMT2-like enzyme for sulfide oxidation from Pseudomonas putida. Can J Microbiol 52:724–730
Shibata H, Suzuki K, Kobayashi S (2007) Menaquinone reduction by an HMT2-like sulfide dehydrogenase from Bacillus stearothermophilus. Can J Microbiol 53:1091–1100
Theissen U, Martin W (2008) Sulfide:quinone oxidoreductase (SQR) from the lugworm Arenicola marina shows cyanide-and thioredoxin-dependent activity. FEBS J 275:1131–1139
Theissen U, Hoffmeister M, Grieshaber M, Martin W (2003) Single eubacterial origin of eukaryotic sulfide:quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol Biol Evol 20:1564–1574
Vande Weghe JG, Ow DW (1999) A fission yeast gene for mitochondrial sulfide oxidation. J Bio Chem 274:13250–13257
Völkel S, Grieshaber MK (1997) Sulphide oxidation and oxidative phosphorylation in the mitochondria of the lugworm Arenicola marina. J Exp Biol 200:83–92
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Wohlgemuth SE, Taylor AC, Grieshaber MK (2000) Ventilatory and metabolic responses to hypoxia and sulphide in the lugworm Arenicola marina (L.). J Exp Biol 203:3177–3188
Zhang ZF, Wang SF, Huo JG, Shao MY, Kang KH (2006) Adaptation of respiratory metabolism to sulfide exposure in Urechis unicinctus. Period Ocean Univ China 36:639–644 (in Chinese with English abstract)
Acknowledgements
We thank Dr. Jianxin Sui for technical assistance in polyclonal antibody preparation. This work is supported by the Natural Science Foundation of China (NSFC) [40776074].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, YB., Zhang, ZF., Shao, MY. et al. Sulfide:quinone Oxidoreductase from Echiuran Worm Urechis unicinctus . Mar Biotechnol 13, 93–107 (2011). https://doi.org/10.1007/s10126-010-9273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-010-9273-3