Abstract
In invertebrates inhabiting hydrothermal vent areas, hypotaurine, a precursor of taurine, is thought to mitigate the toxicity of hydrogen sulfide in vent fluids. Information about hypotaurine synthesis pathways in invertebrates is limited, although two pathways, the cysteamine [2-aminoethanethiol (AET)] pathway and the cysteine sulfinate (CSA) pathway are known in mammals. In this study, we cloned a cDNA encoding AET dioxygenase (ADO), the central enzyme of the AET pathway, from the vent mussel Bathymodiolus septemdierum. In the encoded protein (BsADO), functionally important residues, including metal-binding histidines, are conserved. In maximum likelihood phylogenetic analysis, BsADO clustered with ADOs of other invertebrates. By reverse transcription PCR, BsADO mRNA was detected in all tissues examined at similar levels, suggesting that its function is distinct from that of the CSA pathway, predominantly expressed in the gill. BsADO with a His tag, expressed in Escherichia coli in the presence of Fe2+, converted AET to hypotaurine, but BsADO expressed in the absence of iron exhibited lower activity. BsADO was active from pH 8 to 11, and from 0 °C to 37 °C, with a peak at 20 °C. This is the first functional characterization of ADO in marine invertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Densities and biomasses of deep-sea fauna are generally low, due to limited availability of foods derived from photosynthesis by algae and bacteria. However, dense invertebrate communities occur around deep-sea hydrothermal vents and seeps (Van Dover 2000; Shulze 2019). Such communities are supported by chemosynthetic bacteria, which synthesize organic matter by utilizing chemical energy of components in vent and seep effluents, such as methane, hydrogen, and hydrogen sulfide (Dick 2019). Many invertebrates in vent and seep communities host chemosynthetic bacteria as endosymbionts (Dubilier et al. 2008). Endosymbiosis enables hosts to obtain nutrients without foraging; however, in return, hosts must take up substances required for chemosynthesis by the symbionts (Sogin et al. 2021). This task is especially difficult when symbionts are sulfur-oxidizing bacteria that produce carbohydrates using hydrogen sulfide, because hydrogen sulfide is toxic to respiratory and nervous systems (Wang 2012), and mechanisms by which hosts cope with the toxicity are not well understood. Some proteins, including hemoglobins that bind hydrogen sulfide, have been discovered in siboglinid polychaetes and vesicomyid clams (Childress et al. 1993; Zal et al 1998; 2000; Numoto et al. 2005; Decker et al. 2017); however, such components are known only from a few species (Hourdez and Weber 2005).
Another component that binds hydrogen sulfide is hypotaurine, a precursor of taurine (Nagasaki et al. 2018). Hypotaurine reacts with hydrogen sulfide ion to become thiotaurine, and this reaction is reversible (Fig. 1). As thiotaurine is practically nontoxic, it is a good way to sequester hydrogen sulfide (Pruski et al. 2000; Pruski and Fiala-Médioni 2003; Yancey et al. 2005; Kuroda et al. 2021). In this system, tissues exposed to hydrogen sulfide must accumulate hypotaurine. We have studied mechanisms of hypotaurine accumulation using a deep-sea mussel Bathymodiolus septemdierum, a dominant species in hydrothermal vent areas around Japan (Hashimoto and Okutani 1994; Inoue et al. 2021). This species harbors sulfur oxidizing bacteria in gills (Fujinoki et al. 2012a, b; Ikuta et al. 2016; 2021), and is suitable for experimental purposes because it can be maintained in the laboratory (Fujinoki et al. 2012b). Moreover, it is tough enough to be used for in situ transfer experiments (Koito et al. 2010).
Hypotaurine synthesis pathways. The cysteamine [2-aminoethanethiol (AET)] pathway converts AET, which is generated through the pantothenic acid (PA)/coenzyme A (CoA) metabolic cycle, to hypotaurine by the function of AET dioxygenase (ADO). The cysteine sulfinic acid (CSA) pathway comprises reactions from cysteine to CSA and from CSA to hypotaurine, catabolized by cysteine dioxygenase (CDO) and CSA decarboxylase (CSAD), respectively. Hypotaurine is a precursor of taurine, and also binds to hydrogen sulfide ion to generate thiotaurine
To accumulate hypotaurine, exogenous hypotaurine may be imported from outside cells, or it may be biosynthesized. We have demonstrated that the taurine transporter (TAUT) transports hypotaurine into gill cells, and a GABA transporter (GAT-1) also contributes to raising intracellular hypotaurine concentrations (Inoue et al. 2008; Kinjo et al. 2019). Two pathways in mammals participate in hypotaurine synthesis (Fig. 1). One is the cysteine sulfinic acid (CSA) pathway, and the other is the cysteamine [2-aminoethanethiol (AET)] pathway (Ubuka et al. 2008). Previously, we proposed the existence of the CSA pathway in B. septemdierum by identifying two enzyme genes, cysteine dioxygenase (CDO) and CSA decarboxylase (CSAD), which catalyze the reactions from cysteine to CSA and from CSA to hypotaurine, respectively (Nagasaki et al. 2015). However, there have been no reports of the AET pathway in this species, in which hypotaurine is synthesized from AET by AET dioxygenase (ADO) (Nagasaki et al. 2018). Among marine invertebrates, the only functional evidence for the AET pathway has been the detection of ADO activity in tissue extracts of cephalopods (Kataoka et al. 1988; Matsumoto et al. 2021), although many genomic and transcriptomic sequences have been annotated as ADO genes on the basis of sequence similarity.
In this study, we identified a cDNA encoding ADO from B. septemdierum, and structurally characterized the encoded enzyme (BsADO) by motif and phylogenetic analyses. Subsequently, tissue specificity of its expression was examined by reverse-transcription (RT)–PCR. Finally, BsADO was heterologously expressed in Escherichia coli. Using the recombinant protein, we confirmed its enzymatic activity, and also determined its optimum temperature and pH.
Materials and methods
Mussel samples
Deep-sea mussels B. septemdierum were collected from hydrothermal vent sites on the Myojin Knoll during cruises KY15-07 (on April 27 2015; 32°07.480’N; 139°50.534’E; depth, 1182 m) and KS-18–3 (on April 4–5 2018; 32°06.2202’N; 139°52.1497’E; depth, 1223 m) using the remotely operated vehicle (ROV) Hyper-Dolphin, operated by the research vessels Kaiyo and Shinsei Maru of the Japan Agency of Marine-Earth Science and Technology (JAMSTEC), respectively. Immediately after recovery of the ROV, mantles, gills, feet, adductor muscles, byssus retractor muscles, kidneys, hearts, and gonads were dissected and stored in RNAlater (Thermo Fisher, Waltham, Massachusetts, USA) at −30 °C.
Total RNA extraction and reverse-transcription
Total RNA was extracted from tissues using TRIsure (Bioline, London, UK). Using 500 ng total RNA, cDNA was synthesized using the Superscript III First Strand Synthesis System for RT–PCR (Thermo Fisher) with the oligo dT primer.
Cloning of ADO cDNA
Using the gill cDNA of a mussel collected during cruise KS-18–3 as a template, ADO cDNA was amplified by polymerase chain reaction (PCR) using KOD FX Neo (Toyobo, Osaka, Japan), following the protocol supplied with the enzyme. Amplification was performed using 35 thermal cycles of 98 °C for 10 s, 53 °C for 30 s, and 68 °C for 24 s. Primers used were BsADOf + 40 and BsADOr + 40 (Table 1; Online Resource, Fig. S1), which were designed using the only contig sequence annotated as ADO in a previous RNA-seq analysis (Nagasaki et al. 2015). The amplified product was electrophoresed on a 1.5% agarose gel and the amplified fragment was purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). The sequence of the fragment was determined by direct Sanger sequencing (Azenta, Shinagawa, Japan).
To insert the fragment into a plasmid vector, PCR was performed using the purified fragment as a template, and primers, In-fusion_ADOf and In-fusion_ADOr (Table 1). The PCR conditions were the same as above. The amplified product was electrophoresed on a 1.5% agarose gel and extracted using a QIAEX II Gel Extraction Kit (Qiagen). The extracted fragment was ligated to EcoRI- and NcoI-digested pET28a using the In-Fusion HD Cloning Kit (Takara, Otsu, Japan), transformed into E. coli DH5α, and plated onto Luria–Bertani (LB) plates [tryptone (1% w/v), yeast extract (0.5% w/v), sodium chloride (0.5% w/v), agar (1.5% w/v)] containing 50 μg/mL kanamycin. The resulting plasmid was named pET28a-ADO. After overnight incubation at 37 °C, colonies were picked, and insertion of the target fragment was confirmed by amplification using GoTaq Master Mix (Promega, Madison, WI, USA). Sequence confirmation of pET28a-ADO was conducted by Sanger sequencing (Azenta).
Sequence comparison
A Basic Local Alignment Search Tool for Protein (Blastp) search was performed, using the amino acid sequence encode by the cDNA cloned into the vector as a query against the nonredundant protein sequence database at National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Characterization of the protein sequence was also attempted using Prosite (https://prosite.expasy.org/) with default settings. Based on results of these analyses, the encoded protein is hereafter called B. septemdierum ADO (BsADO). It was aligned with ADO of Mus musculus (MmADO), plant cysteine oxidase of Arabidopsis thaliana (AtPCO), 3-mercaptopropionate dioxygenase of Pseudomonas aeruginosa (SaMDO), and CDO of Homo sapiens (HsCDO) (Online Resource, Table S1) to find functional motifs.
Phylogenetic analysis
Sequences used for phylogenetic analysis were collected from the UniProtKB/Swiss-Prot and NCBI databases. Sequences categorized as either “experimental evidence at protein level” or “experimental evidence at transcript level” were selected (Online Resource, Table S1). A maximum likelihood (ML) molecular phylogenetic tree was constructed using predicted amino acid sequences. Sequences were aligned using MAFFT v7, and gaps were removed using trimAl v1.2. RAxML v7.2.6. with PROTCATWAG model was used for tree construction (Stamatakis 2006), and bootstrap values were calculated based on 1000 replicates.
Reverse-transcription (RT) PCR
Using cDNA of three mussels collected during cruise KY15-07, RT–PCR was performed to examine tissue specificity of BsADO expression. Mantle, gill, foot, adductor muscle, byssus retractor muscle, kidney, heart, and gonad tissues were analyzed. PCR was performed using a KOD FX Neo (Toyobo) following the protocol supplied with the enzyme. Primers used were BsADOf and BsADOr (Table 1; Online Resource, Fig. S1), with 31 thermal cycles of 98 °C for 10 s, 53 °C for 30 s, and 68 °C for 23 s. A partial 18S sequence was also amplified using the primers 18S(313 bp)_F and 18S(313 bp)_R (Table 1), under the same PCR conditions.
Construction of a vector to express BsADO fused with a his-tag
The vector to express BsADO fused with his-tag at the carboxyl terminal was constructed as follows. PCR amplification of the insert was conducted using KOD Plus Neo (Toyobo) following the protocol supplied with the enzyme. pET28a-ADO was used as a template, and pET28a_ADO(useHis)_Infusion_Fw and pET28a_ADO(useHis)_Infusion_Rv (Table 1) were used as primers. Amplification was conducted with 30 cycles of 98 °C for 10 s and 68 °C for 27 s. The amplified fragment was electrophoresed in a 1.2% agarose gel and purified as described above. The purified fragment was ligated to NcoI- and XhoI-digested pET28a using the In-Fusion HD Cloning Kit (Takara). After ligation, absence of sequence mutation was confirmed by Sanger sequencing (Agenta). The resulting expression vector was designated pET28a-ADO-His.
Recombinant expression of ADO
pET28a-ADO-His was transformed into E. coli Rosetta2(DE3)pLysS (Merck, Darmstadt, Germany) and plated onto LB plates containing 25 µg/mL chloramphenicol and 50 µg/mL kanamycin (LBCK). A colony was precultured in 1 mL LBCK medium overnight and subsequently used to inoculate to 100 mL LBCK medium. When the optical density at 600 nm (OD600) reached 0.4 ~ 0.6, isopropyl β-D-thiogalactopyranoside (IPTG) (final concentration, 1 mM) and ammonium iron (II) sulfate (final concentration, 0.1 mM) were added, and cultured overnight at 30 °C. Ammonium iron (II) sulfate was not added to some cultures to examine the effects of iron. Culture media were centrifuged after OD600 measurements, and bacterial pellets were washed in 20 mM Tris–HCl (pH 8.0), and centrifuged again. Washed pellets were suspended in 15 mL of homogenization buffer containing 20 mM Tris–HCl (pH 8.0), 400 mM NaCl, 5 mM imidazole, 0.1% Tween 20, and 1% protease inhibitor cocktail (Fuji Film Wako, Osaka, Japan), and sonicated using an ultrasonic homogenizer (Q125, QSonica, Newtown, CT, USA) (250 W, 70% pulse, 10 cycles of 10 s on and 20 s off) on ice, and centrifuged at 15,000g for 20 min at 4 °C. Supernatants (soluble fraction) and pellets (insoluble fraction) were collected separately and used for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and his-tag-mediated purification.
Purification of recombinant BsADO
The soluble fraction was filtered using 0.22 µm polyethersulfone (PES) filters and applied to Ni-Sepharose 6 Fast Flow (Merck) that was packed in a Poly Prep Chromatography Column (BioRad, Hercules, CA, USA) and pre-equilibrated with 20 mM Tris–HCl (pH 8.0), 400 mM NaCl, and 5 mM imidazole. Subsequently, the column was washed twice with 3 mL wash buffer (20 mM Tris–HCl (pH 8.0), 400 mM NaCl, 20 mM imidazole), and eluted with 3 mL elution buffer containing 20 mM Tris–HCl (pH 8.0), 400 mM NaCl, and 200 mM imidazole. The eluted fraction was dialyzed overnight using cellulose dialysis tubing (MWCO 12,000~14,000) in 10 mM Tris–HCl (pH 8.0) at 4 °C. The dialysate was centrifuged at 4000g for 10 min at 4 °C, and the supernatant was concentrated to 1~1.5 mL using a Vivaspin 20 (MWCO 1000, PES; Sartorius, Goettingen, Germany). The protein concentration was analyzed using a Protein Assay BCA Kit (Nakarai, Kyoto, Japan). The concentrated solution was divided into 1.5 mL microtubes containing aliquots of 50 µL. These were frozen using liquid nitrogen, and stored at −80 °C.
Detecting activity of purified BsADO
Activity of purified BsADO was examined following the methods by Fernandez et al. (2020) with some modifications. Reaction mixture (100 µL), containing 35 mM Tris HCl (pH 7.4), 8 mM AET, 32 µM BsADO expressed with ammonium iron (II) sulfate, and the same reaction mixture without BsADO (negative control) were prepared and incubated for 20 h at 37 °C. At the end of the reaction, norleucine (2.5 nM) was added as an internal standard (Nagasaki et al. 2018), and the reaction was stopped by heating at 99 °C for 10 min. Generation of hypotaurine and its derivatives (taurine and thiotaurine) was measured as described below. All experiments were performed in triplicate.
Enzymatic activity of BsADO expressed with and without iron
To examine the effect of iron in the culture medium, 100 µL of reaction mixture, containing 35 mM Tris HCl (pH 7.4), 8 mM AET, and 5 µM BsADO expressed with ammonium iron (II) sulfate or without were prepared. Incubation and analysis of reaction products were conducted as described above.
Determination of optimum pH
Reaction mixture (100 µL), containing one of the three buffers, 35 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 5.5, 6.0, or 7.0), 35 mM Tris–HCl (pH 7.0, 8.0, or 9.0), or 35 mM Glycine–NaOH (pH 9.0, 10.0, or 11.0), 8 mM AET, and 10 µM BsADO expressed with ammonium iron (II) sulfate, were prepared. Incubation and analysis of reaction products were conducted as described above, but the reaction time was reduced to 30 min because our preliminary experiment indicated that half the reaction products are generated within 30 min (Online Resource, Fig. S2), and comparison at this time enables sensitive detection of differences.
Determination of optimum temperature
Reaction mixture (100 µL), containing 35 mM Glycine–NaOH (pH 9.0), 8 mM AET, and 10 µM BsADO expressed with ammonium iron (II) sulfate, were prepared. Reaction mixtures were incubated for 30 min at 0, 4, 10, 15, 20, 30, or 37 °C. Termination of the reaction and analysis of reaction products were conducted as describe above.
Analyses of hypotaurine, taurine, and thiotaurine
Hypotaurine, taurine, and thiotaurine were measured according to Nagasaki et al. (2018). Briefly, reaction products were vacuum dried, dissolved in methanol/10% ammonia/phenyl isothiocyanate (PITC) (7:2:1) solution, maintained in the dark for 20 min at room temperature, dried again, and stored at −80 °C. For high-performance liquid chromatography (HPLC) analyses, samples were dissolved in 500 µL PicoTag Eluent (Waters, Milford, MA, USA), and applied to a Prominence HPLC system (Shimadzu, Kyoto, Japan) with an ULTRON VX-ODS column (250 mm × 4.6 mm, 5 µm; Shinwa Chemical Industries, Kyoto, Japan). Details of the HPLC system and mobile phases were described in Nagasaki et al. (2018). A representative result of HPLC analysis is provided in Online Resource, Fig. S3.
Results
Nucleotide and amino acid sequences of the deep-sea mussel ADO
A 735-bp cDNA sequence encoding a protein of 244 residues was cloned (Online Resource, Fig. S1). A Blastp search using the encoded protein sequence as query, detected 486 sequences with E-value cut-off of e−20, and none were annotated as proteins other than ADO or ADO-like (on August 17 2022). However, all detected sequences were identified by sequence homology only, and none were accompanied by experimental evidence. Characterization of the protein sequence using Prosite annotated the whole sequence as ADO. Thus, we named the protein encoded by the cloned cDNA, BsADO. The nucleotide sequence of BsADO was deposited in DDBJ/ENA/GenBank under accession number LC730118.
Functional motifs
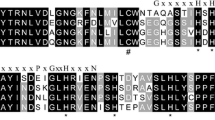
ADO is a thiol oxidase (TDO), a family that also includes plant cysteine oxidase (PCO), 3-mercaptopropionate dioxygenase (MDO), and CDO (York et al. 2021). Thus, we compared the amino acid sequence of BsADO with mouse ADO (MmADO), plant PCO (AtPCO), bacterial MDO (SaMDO), and human CDO (HsCDO) sequences (Online Resource, Table S1; Fig. 2). Two cupin motifs were identified in BsADO as GCRIPLHDHPDMYGFCKVIHG and KCCVITPEFGTYHEIVA (conserved residues in bold). Although the degree of sequence conservation was not high in the cupin motifs (Fig. 2), three histidine residues (His90, His92, and His164) at the reaction center (McCoy et al. 2006) were conserved among the compared TDOs. Glycine residues that are also characteristic of cupin motif 1 are also highly conserved among TDOs. Cysteine residues in or near cupin motifs (Cys109 and Cys154) involved in oxygen delivery (Fernandez et al. 2020), and an ADO-specific glycine residue (Gly 97) were conserved between BsADO and MmADO. In addition, a set of tyrosine and cysteine residues (Tyr183, Cys192, and Tyr194) in a downstream region involved in substrate recognition (White et al. 2020), were also conserved in ADOs and AtPCO.
Alignment of the amino acid sequence of the deep-sea mussel cysteamine dioxygenase (ADO) with sequences of other thiol oxidases (TDOs). Functionally important amino acid residues conserved between BsADO and other TDOs, including histidine residues involved in metal binding (*), cysteine residues involved in oxygen delivery (#), tyrosine and cysteine residues involved in substrate recognition (+), glycine residues that characterize cupin motif 1 (&), and an ADO-specific glycine residue (~) are boxed. Unalignable domains are not shown. BsADO, Bathymodiolus septemdierum ADO; MmADO, Mus musculus ADO; AtPCO, Arabidopsis thaliana plant cysteine oxidase, PaMDO, Pseudomonas aeruginosa mercaptopropionate dioxygenase; HsCDO, Homo sapiens cysteine dioxygenase. Accession numbers of the sequences are indicated in Online Resource, Table S1
Phylogenetic analysis
An ML tree was constructed using ADO sequences accompanying experimental evidence as operational taxonomic units (OTUs) (Fig. 3). In the constructed tree, ADOs formed a clade separate from CDOs and MDO, with a high bootstrap value. BsADO was included in the ADO clade, in which it clustered with invertebrate ADOs. Thus, the identity of BsADO as ADO was confirmed phylogenetically. However, OTUs within the ADO clade were not well resolved.
Maximum likelihood tree constructed using amino acid sequences of cysteamine dioxygenases (ADO), including the deep-sea mussel ADO. ADO sequences, the existence of which were confirmed at protein or transcript levels, were used for analysis (Online Resource, Table S1). Some cysteine dioxygenase (CDO) and mercaptopropionate dioxygenase (MDO) sequences were added for comparison. The tree was constructed using RAxML after alignment with MAFFT v7. Bootstrap values were calculated based on 1000 replicates, and indicated at nodes where the value was > 70
Tissue specificity of gene expression
Levels of BsADO transcripts in eight major tissues were compared by RT–PCR (Fig. 4; Online Resource, Fig. S4). The BsADO gene is expressed in all tissues examined.
Tissue specificity of mRNA expression of the deep-sea mussel cysteamine dioxygenase (ADO). Total RNA (500 µg) was reverse transcribed using Superscript III (Thermo Fisher) and the Oligo dT primer, and 31 cycles of amplification were conducted using KOD FX Neo (Toyobo). Primers BsADOf /BsADOr and 18S(313 bp)_F/18S(313 bp)_R (Table 1), were used for amplification of partial ADO and 18SrRNA (internal control) sequences, respectively. Amplified products were electrophoresed in a 1.5% agarose gel. Expected fragment sizes were 735 bp (ADO) and 313 bp (18S rRNA). Gene Ladder Wide 1 (Nippon Gene) was loaded on both sides, and sizes of markers (bp) near the targets are indicated at the right. M, mantle; G, gill; F, foot; AM, adductor muscle; BM, byssus retractor muscle; K, kidney; H, heart; Go, gonad. Original pictures are shown in Online Resource, Fig. S4
Heterologous expression of BsADO in E. coli and purification
BsADO, with a his-tag at the C-terminus was successfully expressed primarily in the soluble fraction of bacterial cells. It was purified by Ni-affinity chromatography followed by dialysis and centrifugal concentration (Online Resource, Fig. S5). The resulting protein, with a predicted molecular weight of 28,180.34 Da, was confirmed by SDS-PAGE (Online Resource, Fig. S5). From a 100-mL culture, 320 µmol recombinant ADO with his-tag was obtained.
Activity of purified BsADO and the effect of iron
Activity of the purified BsADO was examined by reacting it with AET, and analyzing the amount of hypotaurine, taurine, and thiotaurine. Although hypotaurine is the direct product of the reaction of ADO, it tends to be converted to taurine and thiotaurine (Fig. 1) spontaneously without specific enzymes (Nagasaki et al. 2018). Therefore, the result was presented as the sum of the three substances (reaction products). Even just after the start of the reaction, small amounts of reaction products were already detected, but they increased after 20 h (Fig. 5). When the enzyme was not added to the reaction mixture (negative control), reaction products were below the detection limit, even after 20 h. Thus, the activity of BsADO to convert AET to hypotaurine was demonstrated and the identity of BsADO was functionally confirmed.
Activities of the deep-sea mussel cysteamine dioxygenase (BsADO) expressed in Escherichia coli. BsADO with a his tag, expressed in E. coli in the presence of Fe2+, and purified by affinity chromatography and dialysis, was reacted with cysteamine (AET) for 20 h at 37 ˚C. Reaction products (hypotaurine, taurine, and thiotaurine) were analyzed by reverse-phase high-performance liquid chromatography (HPLC) according to Nagasaki et al. (2018), and reported as the sum of the three compounds. For a negative control, the same reaction mixture without enzyme was tested. tr, trace level. Data were acquired in triplicate and are presented as means and standard deviations
Subsequently, activities of BsADO expressed with/without iron were compared (Fig. 6). BsADO expressed with iron generated six times more reaction products than that expressed without iron. Thus, addition of iron to the culture medium is important to obtain proper activity of ADO.
Comparison of activities of the deep-sea mussel cysteamine dioxygenase (BsADO) expressed in Escherichia coli in the presence or absence of Fe2+. BsADO with a his tag, expressed in E. coli in the presence or absence of Fe2+, and purified by affinity chromatography and dialysis, was reacted with cysteamine (AET) in the absence of Fe2+ for 20 h at 37 °C. Reaction products (hypotaurine, taurine, and thiotaurine) were analyzed by reverse-phase high-performance liquid chromatography (HPLC) according to Nagasaki et al. (2018), and indicated as the sum of the three compounds. Data were acquired in triplicate and are presented as means and standard deviations. ND, not detected
Optimum pH and temperature
The optimum pH of BsADO was 9.0 (Fig. 7). BsADO activity was higher from pH 8 to 11 than from pH 5.5 to 7. Subsequently, optimum reaction temperature was examined from 0 °C to 37 °C, and 20 °C was optimal (Fig. 8). Even at 0 °C and 37 °C, 44% and 54% of reaction products at the optimum temperature, respectively, were detected, indicating that this enzyme can function throughout the temperature range tested in this study.
pH preference of the deep-sea mussel cysteamine dioxygenase (BsADO) expressed in Escherichia coli. BsADO with a his tag, expressed in E. coli in the presence of Fe2+, and purified by affinity chromatography and dialysis, was reacted with cysteamine (AET) for 30 min at 37 °C, and reaction products (hypotaurine, taurine, and thiotaurine) were analyzed by reverse-phase high-performance liquid chromatography (HPLC) according to Nagasaki et al. (2018). These are indicated as the sum of the three compounds. For pH adjustment, 2-(N-morpholino)ethanesulfonic acid (MES), Tris–HCl, and Glycine–NaOH buffers were used for pH 5.5–7.0, pH 7.0–9.0, and pH 9.0–11.0, respectively. Data were acquired in triplicate and are presented as means and standard deviations
Temperature preference of the deep-sea mussel cysteamine dioxygenase (BsADO) expressed in Escherichia coli. BsADO with a his tag, expressed in E. coli in the presence of Fe2+, and purified by affinity chromatography and dialysis, was reacted with cysteamine (AET) for 30 min at 0–37 °C. Reaction products (hypotaurine, taurine, and thiotaurine) were analyzed by reverse-phase high-performance liquid chromatography (HPLC) according to Nagasaki et al. (2018), and reported as the sum of the three compounds. Data were acquired in triplicate and are presented as means and standard deviations
Discussion
In this study, we cloned a cDNA encoding an ADO-like protein from the deep-sea mussel, B. septemdierum. We named the encoded protein BsADO, based on high homology with ADO proteins of other organisms. The start point of the coding region was determined to be the first methionine codon appeared in the RNA-seq contig (Online Resource, Fig. S1). Although its upstream 29-bp sequence does not contain an in-frame stop codon, its homologs of oysters and a scallop (XM_048922430, XM_022470558, and XM_033901629) contain one just upstream of the corresponding methionine codon.
BsADO was suggested to be a functional thiol oxidase (TDO) due to its cupin motifs (Fig. 2). Cupin motif 1, GX5HXHX4EX5G, was clearly identified as GCRIPLHDHPDMYGFCKVIHG. Substitution of E by G is common among ADOs (Stipanuk et al. 2011). In contrast, cupin motif 2, which has diversified in TDOs from the original definition (GX5PXGX2HX3N), was identified following Dominy et al. (2007) (Fig. 2). Although amino acid sequences of the two cupin motifs of BsADO are less conserved among TDOs, as has been reported in ADOs of other organisms (Dominy et al. 2007), three histidine residues (His90, His92, and His164) that form the active center through coordinate bonding to Fe2+ (McCoy et al. 2006) are conserved (Fig. 2). In addition, two functional cysteine residues in and near cupin motifs (Cys109 and Cys154), which are thought to regulate delivery of oxygen in mouse ADO (Fernandez et al. 2020), are conserved in BsADO. Moreover, one cysteine and two tyrosine residues (Tyr183, Cys192, and Tyr194) involved in substrate recognition (White et al. 2020) are also present in ADOs and AtPCO, which is plant specific, but its similarity to animal ADOs has been reported (Holdsworth and Gibbs 2020). Conservation of such residues (Fig. 2) suggests that BsADO is a functional ADO. Phylogenetic analysis with known ADOs and some MDOs and CDOs located the encoded protein in a cluster of invertebrate ADOs in the ADO clade (Fig. 3). The high bootstrap values of the ADO clade and the invertebrate ADO cluster confirm that BsADO belongs to the ADO lineage. The order of branching within the ADO clade did not agree with organismal phylogeny, which may reflect an insufficiency of amino acids that are informative for tree construction, as ADO sequences are generally short, with low sequence conservation, as has been discussed for a related enzyme, CDO (Kuroda et al. 2021).
Expression of the BsADO gene was detected in all tissues examined (Fig. 4). The wide expression of BsADO contrasts with the genes, BsCDO and BsCSAD, involved in the CSA pathway, expressed predominantly in the gill (Nagasaki et al. 2015). The AET pathway may serve to maintain hypotaurine levels in various tissues, and the CSA pathway may respond to the special demand for hypotaurine in the gill, which is the major site of hydrogen sulfide uptake and delivery to symbiotic bacteria. Expression of ADO in various tissues is consistent with the fact that AET, the substrate of ADO, is generated in many tissues through the metabolic cycle of coenzyme A, which is involved in essential cellular functions (Gout 2018; Atallah et al. 2020). ADO may be also involved in regulation of the level of AET, which is involved in various reactions in the body, including oxidative stress resistance (Jeitner and Lawrence 2001; Besouw et al. 2013). Therefore, the AET pathway may not be suitable to respond to high hypotaurine demand in the gill.
Enzymatic activity of BsADO was demonstrated by expressing it in E. coli. BsADO protein purified through Ni-affinity chromatography converted AET to hypotaurine and its downstream products, taurine and thiotaurine; thus, the identity of the BsADO was functionally confirmed (Fig. 5). We could not detect reduction of AET after the reaction because AET is not detectable by standard amino acid analytical methods. However, it is evident that the reaction products were derived from AET because the reaction mixture, consisting of only buffer, AET, and dialyzed enzyme, does not contain any other compounds that could be converted to hypotaurine.
We added ammonium iron (II) sulfate to the culture medium at the time of IPTG induction because incorporation of divalent iron into ADO has been reported (Fernandez et al. 2020). In the experiment in which iron addition was omitted, BsADO activity decreased to one sixth (Fig. 6). Thus, incorporation of divalent iron during protein expression is important for BsADO activity. In our preliminary experiment, addition of the same compound to the reaction mixture was ineffective, suggesting that divalent iron is not a cofactor of the enzymatic reaction, and that it must be incorporated into BsADO in expressing cells to form the proper enzyme structure as mentioned above.
Recombinant BsADO was optimally active from pH 8 to 10, and less active under neutral to acidic conditions (Fig. 7). The optimum pH range has been also examined for recombinant mouse MmADO (Dominy et al. 2007). Although specific activity cannot be compared between MmADO and BsADO due to differences in experimental methods, the optimum pH range is almost the same. Therefore, a pH preference near 9 may be a common characteristic of animal ADOs. It is also possible that the pH influenced disulfide bond formation in ADO (Wang et al. 2020) and/or its substrate, AET (Atallah et al. 2020). Mussels may maintain tissue pH in the slightly alkaline range, although we have not measured internal pH of mussels. In shallow-water mussels, hemolymph pH is influenced by ambient pH (Mangan et al. 2019). At Myojin Knoll vent sites, where specimens for this study were collected, B. septemdierum colonies occupy locations where they are not exposed directly to vent effluent (Inoue et al. 2021), and pH of the water inside the colonies, measured during previous research cruise, was 7.28 ± 0.90 (unpublished observation during cruise NT14-06, 2014). Thus, regulation of tissue pH at weakly alkaline levels may not be a severe challenge. Mussels may decide where to attach based on water pH, in addition to sulfide levels. Weakly alkaline conditions may also be advantageous for both the mussel and its symbionts because such conditions enable hydrogen sulfide to ionize, although B. septemdierum is reportedly able to adapt to acidic conditions (Rossi and Tunnicliffe 2017). It will be interesting to learn how mussels regulate tissue pH under acidic conditions.
BsADO was active under at temperatures from 0 °C to 37 °C (Fig. 8). This is advantageous for inhabiting hydrothermal vents where water temperatures fluctuate. The temperature of vent fluids exceeds 200 °C, but that of deep seawater otherwise is 1–3 °C (Gamo et al. 2006). As mentioned above, mussels avoid direct exposure to vent fluids; thus, the temperature around B. septemdierum colonies is around 5 °C (Nakamura-Kusakabe et al. 2016). They can survive at 20 °C or higher under experimental conditions, but such temperatures seem stressful (Boutet et al. 2009; Nakamura-Kusakabe et al. 2016), and long-term culture of this species is not possible at 30 °C or higher (unpublished observations). However, they may be exposed to such temperatures accidentally by sudden change of the course of vent fluid, which may be a reason why BsADO functions at 37 °C.
In summary, we isolated ADO cDNA from the deep-sea mussel, B. septemdierum, and its identity was functionally confirmed. This is the first report of a molluskan ADO with functional evidence, although ADO sequences have been registered in many invertebrates, including mollusks, through annotation of genome and transcriptome sequences. The presence of functional ADO implies the existence of the AET pathway to synthesize hypotaurine and taurine, and the AET and CSA pathways are thought to serve different functions. Our findings will advance the understanding of hypotaurine metabolism, which mitigates hydrogen sulfide toxicity, and which also synthesizes taurine, an important compound for homeostasis and stress responses.
References
Atallah C, Charcosset C, Greige-Gerges H (2020) Challenges for cysteamine stabilization, quantification, and biological effects improvement. J Pharm Anal 10:499–516
Besouw M, Masereeuw R, van den Heuvel L, Levtchenko E (2013) Cysteamine: an old drug with new potential. Drug Discov Today 18:785–792
Boutet I, Tanguy A, Le Guen D, Piccino P, Hourdez S, Legendre P, Jollivet D (2009) Global depression in gene expression as a response to rapid thermal changes in vent mussels. Proc Biol Sci 276:3071–3079
Childress JJ, Fisher CR, Favuzzi JA, Arp AJ, Oros DR (1993) The role of a zinc-based, serum-borne sulphide binding component in the uptake and transport of dissolved sulphide by the chemoautotrophic symbiont-containing clam Calyprogena elongata. J Exp Biol 179:131–158
Decker C, Zorn N, Le Bruchec J, Caprais JC, Potier N, Leize-Wagner E, Lallier FH, Olu K, Andersen AC (2017) Can the hemoglobin characteristics of vesicomyid clam species influence their distribution in deep-sea sulfide-rich sediments? A case study in the Angola Basin. Deep-Sea Res II 142:219–232
Dick GJ (2019) The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally. Nat Rev Microbiol 17:271–283
Dominy JE, Simmons CR, Hirschberger LL, Hwang J, Coloso RM, Stipanuk MH (2007) Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J Biol Chem 282:25189–25198
Dubilier N, Bergin C, Lott C (2008) Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol 6:725–740
Fernandez RL, Dillon SL, Stipanuk MH, Fox BG, Brunold TC (2020) Spectroscopic investigation of cysteamine dioxygenase. Biochemistry 59:2450–2458
Fujinoki M, Koito T, Fujiwara Y, Kawato M, Tada Y, Hamasaki K, Jimbo M, Inoue K (2012a) Whole-mount fluorescence in situ hybridization to visualize symbiotic bacteria in the gills of deep-sea mussels. Aquat Biol 14:135–140
Fujinoki M, Koito T, Nemoto S, Kitada M, Yamaguchi Y, Hyodo S, Numanami H, Miyazaki N, Inoue K (2012b) Comparison of the amount of thiotrophic symbionts in the deep-sea mussel Bathymodiolus septemdierum under different sulfide levels using fluorescent in situ hybridization. Fish Sci 78:139–146
Gamo T, Ishibashi J, Tsunogai U, Okamura K, Chiba H (2006) Unique geochemistry of submarine hydrothermal fluids from arc˗back-arc setting of the western Pacific. In: Christie DM et al (eds) Back-arc spreading systems: geological,biological, chemical, and physical interactions, geophysical monograph series. American Geophysical Union, Washington, D. C, pp 147–161
Gout I (2018) Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem Soc Trans 46:721–728
Hashimoto J, Okutani T (1994) Four new mytilid mussels associated with deepsea chemosynthetic communities around Japan. Venus 53:61–83
Holdsworth MJ, Gibbs DJ (2020) Comparative biology of oxygen sensing in plants and animals. Curr Biol 30:R362–R369
Hourdez S, Weber RE (2005) Molecular and functional adaptations in deep-sea hemoglobins. J Inorg Biochem 99:130–141
Ikuta T, Takaki Y, Nagai Y, Shimamura S, Tsuda M, Kawagucci S, Aoki Y, Inoue K, Teruya M, Satou K, Teruya K, Shimoji M, Tamotsu H, Hirano T, Maruyama T, Yoshida T (2016) Heterogeneous composition of key metabolic gene clusters in a vent mussel symbiont population. ISME J 10:990–1001
Ikuta T, Amari Y, Tame A, Takaki Y, Tsuda M, Iizuka R, Funatsu T, Yoshida T (2021) Inside or out? Clonal thiotrophic symbiont populations occupy deep-sea mussel bacteriocytes with pathways connecting to the external environment. ISME Commun 1:38
Inoue K, Tsukuda K, Koito T, Miyazaki Y, Hosoi M, Kado R, Miyazaki N, Toyohara H (2008) Possible role of a taurine transporter in the deep-sea mussel Bathymodiolus septemdierum in adaptation to hydrothermal vents. FEBS Lett 582:1542–1546
Inoue K, Onitsuka Y, Koito T (2021) Mussel biology: from the byssus to ecology and physiology, including microplastic ingestion and deep-sea adaptations. Fish Sci 87:761–771
Jeitner TM, Lawrence DA (2001) Mechanisms for the cytotoxicity of cysteamine. Toxicol Sci 63:57–64
Kataoka H, Ohishi K, Imai J, Mukai M (1988) Distribution of cysteamine dioxygenase in animal tissues. Agric Biol Chem 52:1611–1613
Kinjo A, Sassa M, Koito T, Suzuki M, Inoue K (2019) Functional characterization of the GABA transporter GAT-1 from the deep-sea mussel Bathymodiolus septemdierum. Comp Biochem Physiol A Mol Integr Physiol 227:1–7
Koito T, Morimoto S, Toyohara H, Yoshida T, Jimbo M, Maruyama T, Miyazaki N, Inoue K (2010) Decline in taurine transporter mRNA and thioautotrophic bacterial 16S rDNA levels after transplantation of the hydrothermal-vent mussel Bathymodiolus septemdierum to a non-vent position. Cah Biol Mar 51:429–433
Kuroda M, Nagasaki T, Koito T, Hongo Y, Yoshida T, Maruyama T, Tsuchida S, Nemoto S, Inoue K (2021) Possible roles of hypotaurine and thiotaurine in the vesicomyid clam Phreagena okutanii. Biol Bull 240:34–40
Mangan S, Wilson RW, Findlay HS, Lewis C (2019) Acid-base physiology over tidal periods in the mussel Mytilus edulis: size and temperature are more influential than seawater pH. Proc Biol Sci 286:20182863
Matsumoto T, Akita M, Ogawa M, Goto T (2021) Evaluation of taurine biosynthesis in the livers of the spear squid Heterololigo bleekeri and the swordtip squid Uroteuthis edulis. Fish Sci 87:717–725
McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN (2006) Structure and mechanism of mouse cysteine dioxygenase. Proc Natl Acad Sci USA 103:3084–3089
Nagasaki T, Hongo Y, Koito T, Kusakabe-Nakamura I, Shimamura S, Takaki Y, Yoshida T, Maruyama T, Inoue K (2015) Cysteine dioxygenase and cysteine sulfinate decarboxylase genes of the deep-sea mussel Bathymodiolus septemdierum: possible involvement in hypotaurine synthesis and adaptation to hydrogen sulfide. Amino Acids 47:571–578
Nagasaki T, Koito T, Nemoto S, Ushio H, Inoue K (2018) Simultaneous analysis of free amino acids and taurine-related compounds in deep-sea mussel tissues using reversed-phase HPLC. Fish Sci 84:127–134
Nakamura-Kusakabe I, Nagasaki T, Kinjo A, Sassa M, Koito T, Okamura K, Yamagami S, Yamanaka T, Tsuchida S, Inoue K (2016) Effect of sulfide, osmotic, and thermal stresses on taurine transporter mRNA levels in the gills of the hydrothermal vent-specific mussel Bathymodiolus septemdierum. Comp Biochem Physiol A Mol Integr Physiol 191:74–79
Numoto N, Nakagawa T, Kita A, Sasayama Y, Fukumori Y, Miki K (2005) Structure of an extracellular giant hemoglobin of the gutless beard worm Oligobrachia mashikoi. Proc Natl Acad Sci USA 102:14521–14526
Pruski AM, Fiala-Médioni A (2003) Stimulatory effect of sulphide on thiotaurine synthesis in three hydrothermal-vent species from the East Pacific Rise. J Exp Biol 206:2923–2930
Pruski AM, Fiala-Médioni A, Fisher CR, Colomines JC (2000) Composition of free amino acids and related compounds in invertebrates with symbiotic bacteria at hydrocarbon seeps in the Gulf of Mexico. Mar Biol 136:411–420
Rossi GS, Tunnicliffe V (2017) Trade-offs in a high CO2 habitat on a subsea volcano: condition and reproductive features of a bathymodioline mussel. Mar Ecol Prog Ser 574:49–64
Shulze A (2019) Cold seep ecosystems. In: Cochran JK et al (eds) Encyclopedia of ocean sciences. Third Edition, Elsevier New York, pp 677–683
Sogin EM, Kleiner M, Borowski C, Gruber-Vodicka HR, Dubilier N (2021) Life in the dark: phylogenetic and physiological diversity of chemosynthetic symbioses. Annu Rev Microbiol 75:695–718
Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stipanuk MH, Simmons CR, Karplus PA, Dominy JE (2011) Thiol dioxygenases: unique families of cupin proteins. Amino Acids 41:91–102
Ubuka T, Okada A, Nakamura H (2008) Production of hypotaurine from L-cysteinesulfinate by rat liver mitochondria. Amino Acids 35:53–58
Van Dover CL (2000) The ecology of deep-sea hydrothermal vents. Princeton University Press, New Jersey
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896
Wang Y, Davis I, Chan Y, Naik SG, Griffith WP, Liu A (2020) Characterization of the nonheme iron center of cysteamine dioxygenase and its interaction with substrates. J Biol Chem 295:11789–11802
White MD, Dalle Carbonare L, Puerta ML, Iacopino S, Edwards M, Dunne K, Pires E, Levy C, McDonough MA, Licausi F (2020) Structures of Arabidopsis thaliana oxygen-sensing plant cysteine oxidases 4 and 5 enable targeted manipulation of their activity. Proc Natl Acad Sci USA 117:23140–23147
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
York NJ, Lockart MM, Sardar S, Khadka N, Shi W, Stenkamp RE, Zhang J, Kiser PD, Pierce BS (2021) Structure of 3-mercaptopropionic acid dioxygenase with a substrate analog reveals bidentate substrate binding at the iron center. J Biol Chem 296:100492
Zal F, Leize E, Lallier FH, Toulmond A, Van Dorsselaer A, Childress JJ (1998) S-Sulfohemoglobin and disulfide exchange: the mechanisms of sulfide binding by Riftia pachyptila hemoglobins. Proc Natl Acad Sci USA 95:8997–9002
Zal F, Leize E, Oros DR, Hourdez S, Dorsselaer AV, Childress JJ (2000) Haemoglobin structure and biochemical characteristics of the sulphide-binding component from the deep-sea clam Calyptogena magnifica. Cah Biol Mar 41:413–423
Acknowledgements
This study was partially supported by the Interdisciplinary Collaborative Research Program of the Atmosphere and Ocean Research Institute, the University of Tokyo (for TK, no. 131). The authors thank the operating team of the remotely operated vehicle Hyper-Dolphin, and also the crew of the RV Kaiyo and RV Shinsei Maru of Japan Agency for Marine-Earth Science and Technology (JAMSTEC).
Funding
Open access funding provided by The University of Tokyo. Atmosphere and Ocean Research Institute, The University of Tokyo,Interdisciplinary Collaborative Research Program no. 131, Tomoko Koito.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoda, K., Takagi, T., Koito, T. et al. Heterologous expression and functional characterization of cysteamine dioxygenase from the deep-sea mussel Bathymodiolus septemdierum. Fish Sci 89, 387–397 (2023). https://doi.org/10.1007/s12562-023-01674-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-023-01674-w