Abstract
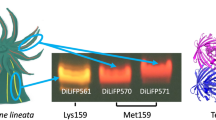
A novel orange fluorescent protein (OFP) was cloned from the tentacles of Cnidarian tube anemone Cerianthus sp. It consists of 222 amino acid residues with a calculated molecular mass of 25.1 kDa. A BLAST protein sequence homology search revealed that native OFP has 81% sequence identity to Cerianthus membranaceus green fluorescent protein (cmFP512), 38% identity to Entacmaea quadricolor red fluorescent protein (eqFP611), 37% identity to Discosoma red fluorescent protein (DsRed), 36% identity to Fungia concinna Kusabira-orange fluorescent protein (KO), and a mere 21% identity to green fluorescent protein (GFP). It is most likely that OFP also adopts the 11-strand β-barrel structure of fluorescent proteins. Spectroscopic analysis indicated that it has a wide absorption spectrum peak at 548 nm with two shoulders at 487 and 513 nm. A bright orange fluorescence maximum at 573 nm was observed when OFP was excited at 515 nm or above. When OFP was excited well below 515 nm, a considerable amount of green emission maximum at 513 nm was also observed. It has a fluorescence quantum yield (Φ) of 0.64 at 25°C. The molar absorption coefficients (ɛ) of folded OFP at 278 and 548 nm are 47,000 and 60,000 M-1−1 • cm-1−1, respectively. Its fluorescent brightness (ɛ Φ) at 25°C is 38,400 M−1-1 • cm−1-1. Like other orange-red fluorescent proteins, OFP is also tetrameric. It was readily expressed as soluble protein in Escherichia coli at 37°C, and no aggregate was observed in transfected HeLa cells under our experimental conditions. Fluorescent intensity of OFP is detectable over a pH range of 3 to 12.





Similar content being viewed by others
References
Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A (2002) An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA 99, 12651–12656
Baird GS, Zacharias DA, Tsien RY (2000) Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci USA 97, 11984–11989
Bevis BJ, Glick BS (2002) Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat Biotechnol 20, 83–87
Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY (2002) A monomeric red fluorescent protein. Proc Natl Acad Sci USA 99, 7877–7882
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prashers DC (1994) Green fluorescent protein as a marker for gene expression. Science 263, 802–805
Chudakov DM, Lukyanov SA, Lukyanov KA (2005) Fluorescent proteins as a toolkit for in vivo imaging. Trends Biochem 23, 605–613
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38
Crameri A, Whitehorn EA, Tate E, Stemmer WP (1996) Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol 14, 315–319
Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY (1995) Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20, 448–455
Delagrave S, Hawtin RE, Silva CM, Yang MM, Youvan, DC (1995) Red-shifted excitation mutants of the green fluorescent protein. Biotechnology (NY) 13, 151–154
Drew DE, von Heijne G, Nordlund P, de Gier JW (2001) Green fluorescent protein as an indicator to monitor membrane protein overexpression in Escherichia coli. FEBS Lett 507, 220–224
Ehrig T, O’Kane DJ, Prendergast FG (1995) Green-fluorescent protein mutants with altered fluorescence excitation spectra. FEBS Lett 367, 163–166
Fery-Forgues S, Lavabre D (1999) Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationary products. J Chem Ed 76, 1260–1264
Fliegel L, Burns K, MacLennan DH, Reithmeier RAF, Michalak M (1989) Molecular cloning of the high affinity calcium-binding protein (Calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 264, 21522–21528
Fradkov AF, Chen Y, Ding L, Barsova EV, Matz MV, Lukyanov SA (2000) Novel fluorescent protein from Discosoma coral and its mutants possesses a unique far-red fluorescence. FEBS Lett 479, 127–130
Gavin P, Devenish J, Prescott M (2002) An approach for reducing unwanted oligomerisation of DsRed fusion proteins. Biochem Biophys Res Commun 298, 707–713
Gerdes HH, Kaether C (1996) Green fluorescent protein: applications in cell biology. FEBS Lett 389, 44–47
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182, 319–326
Gurskaya NG, Fradkov AF, Terskikh A, Matz MV, Labas YA, Martynov VI, Yanushevich, YG, Lukyanov KA, Lukyanov SA (2001) GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett 507, 16–20
Heim R, Tsien RY (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol 6, 178–182
Heim R, Prasher DC, Tsien RY (1994) Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA 91, 12501–12504
Heim R, Cubitt AB, Tsien RY (1995) Improved green fluorescence. Nature 373, 663–664
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59
Hoffman RM (2005) Advantages of multi-color fluorescent proteins for whole-body and in vivo cellular imaging. J Biomed Opt 10, 41202–41212
Inouye S, Tsuji FI (1994) Aequorea green fluorescent protein. Expression of the gene and fluorescence characteristics of the recombinant protein. FEBS Lett 341, 277–280
Ip DTM, Chan SH, Allen MD, Bycroft M, Wan DCC, Wong KB (2003) Crystallization and preliminary crystallographic analysis of a novel orange fluorescent protein from the Cnidaria tube anemone Cerianthus sp. Acta Cryst D60, 340–341
Kalderon D, Roberts BL, Richardson WD, Smith AE (1984) A short amino acid sequence able to specify nuclear location. Cell 39, 499–509
Karasawa S, Araki T, Nagai T, Mizuno H, Miyawaki A (2004) Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem J 381, 307–312
Kendall JM, Badminton MN (1998) Aequorea victoria bioluminescence moves into an exciting new era. Trends Biotechnol 16, 216–224
Labas YA, Gurskaya NG, Yanushevich YG, Fradkov AF, Lukyanov KA, Lukyanov SA, Matz MV (2002) Diversity and evolution of the green fluorescent protein family. Proc Natl Acad Sci USA 99, 4256–4261
Lanford RE, Kanda P, Kennedy RC (1986) Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell 46, 575–582
Lippincott-Schwartz J, Patterson GH (2003) Development and use of fluorescent protein markers in living cells. Science 300, 87–91
Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA (1999) Fluorescent proteins from non-bioluminescent Anthozoa species. Nat Biotechnol 17, 969–973
Matz MV, Lukyanov KA, Lukyanov SA (2002) Family of the green fluorescent protein: journey to the end of the rainbow. Bioessays 24, 953–959
Mitra RD, Silva CM, Youvan DC (1996) Fluorescence resonance energy transfer between blue-emitting and red-shifted excitation derivatives of the green fluorescent protein. Gene 173, 13–17
Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca2+2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887
Morin JG, Hastings JW (1971) Biochemistry of the bioluminescence of colonial hydroids and other coelenterates. J Cell Physiol 77, 305–312
Morise H, Shimomura O, Johnson FH, Winant J (1974) Intermolecular energy transfer in the bioluminescent system. Biochemistry 13, 2656–2662
Munro S, Pelham HRB (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907
Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ (1996) Crystal structure of the Aequorea victoria green fluorescent protein. Science 273, 1392–1395
Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4, 2411–2423
Pelham HRB (1996) The dynamic organisation of the secretory pathway. Cell Struct Funct 21, 413–419
Petersen J, Wilmann PG, Beddoe T, Oakley AJ, Devenish RJ, Prescott M, Rossjohn J (2003) The 20–Å crystal structure of eqFP611, a far red fluorescent protein from the sea anemone Entacmaea quadricolor. J Biol Chem 278, 44626–44631
Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111, 229–233
Sacchetti A, Scbramaniam V, Jovin TM, Alberti S (2002) Oligomerization of DsRed is required for the generation of a functional red fluorescent chromophore. FEBS Lett 525, 13–19
Schmid JA, Neumeier H (2005) Evolutions in science triggered by green fluorescent protein (GFP). Chem BioChem 6, 1–9
Shagin DA, Barsova EV, Yanushevich YG, Fradkov AF, Lukyanov KA, Labas YA, Ugalde JA, Meyer A, Nunes JM, Widder EA, Lukyanov SA, Matz MV (2004) GFP-like proteins as ubiquitous Metazoan superfamily: evolution of functional features and structural complexity. Mol Biol Evol 21, 841–850
Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp red fluorescent protein. Nat Biotechnol 12, 1256–1272
Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan Aequorea. J Cell Comp Physiol 59, 223–239
Thomson CM, Ward WW (2004) Fluorescent Proteins: Technologies, Applications, and Opportunities for Drug Discovery. (Drug and Market Development Publications, Westborough, Massachusetts)
Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67, 509–544
Van Roessel P, Brand AH (2002) Imaging into the future: visualizing gene expression and protein interactions with fluorescent proteins. Nat Cell Biol 4, E15–E20
Verkhusha VV, Lukyanov KA (2004) The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nat Biotechnol 22, 289–296
Wachter RM, Elsliger M-A, Kallio K, Hanson GT, Remington SJ (1998) Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Structure 6, 1267–1277
Wall MA, Socolich M, Ranganathan R (2000) The structural basis for red fluorescence in the tetrameric GFP homolog DsRed. Nat Struct Biol 7, 1133–1138
Wang S, Hazelrigg T (1994) Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature 369, 400–403
Wiedenmann J, Schenk A, Röcker C, Girod A, Spindler K-D, Nienhaus GU (2002) A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc Natl Acad Sci USA 99, 11646–11651
Wiedenmann J, Ivanchenko S, Oswald F, Nienhaus GU (2004) Identification of GFP-like proteins in nonbioluminescent, Azooxanthellate Anthozoa opens new perspectives for bioprospecting. Mar Biotechnol 6, 270–277
Yang F, Moss LG, Philips GN Jr (1996) The molecular structure of green fluorescent protein. Nat Biotechnol 14, 1246–1251
Yanushevich YG, Staroverov DB, Savitsky AP, Fradkov AF, Gurskaya NG, Bulina ME, Lukyanov KA, Lukyanov SA (2002) A strategy for the generation of non-aggregating mutants of Anthozoa fluorescent proteins. FEBS Lett 511, 11–14
Yarbrough D, Wachter RM, Kallio K, Matz MV, Remington SJ (2001) Refined crystal structure of DsRed, a red fluorescent protein from coral, at 20–Å resolution. Proc Natl Acad Sci USA 98, 462–467
Zimmer M (2002) Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem Rev 102, 759–781
Acknowledgments
This research was supported by RGC Earmarked Grant CUHK4571/05M and Direct Grant from CUHK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ip, D.TM., Wong, KB. & Wan, D.CC. Characterization of Novel Orange Fluorescent Protein Cloned from Cnidarian Tube Anemone Cerianthus sp.. Mar Biotechnol 9, 469–478 (2007). https://doi.org/10.1007/s10126-007-9005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9005-5