Abstract
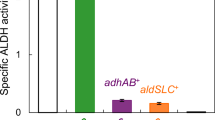
In previous and present studies, four enzymes (GCD1, GCD3, GCD4, and MQO1) have been found to act as lactose-oxidizing enzymes of Pseudomonas taetrolens. To investigate whether the four enzymes were the only lactose-oxidizing enzymes of P. taetrolens, we performed the inactivation of gcd1, gcd3, gcd4, and mqo1 genes in P. taetrolens. Compared to the wild-type strain, the lactobionic acid (LBA)-producing ability of P. taetrolens ∆gcd1 ∆gcd3 ∆gcd4 ∆mqo1 was only slightly decreased, implying that P. taetrolens possesses more lactose-oxidizing enzymes. Interestingly, the four lactose-oxidizing enzymes were all pyrroloquinoline quinone (PQQ)-dependent. To identify other unidentified lactose-oxidizing enzymes of P. taetrolens, we prevented the synthesis of PQQ in P. taetrolens by inactivating the genes related to PQQ synthesis such as pqqC, pqqD, and pqqE. Surprisingly, all three knocked-out strains were unable to convert lactose to LBA, indicating that all lactose-oxidizing enzymes in P. taetrolens were inactivated by eliminating PQQ synthesis. In addition, external PQQ supplementation restored the LBA production ability of P. taetrolens ∆pqqC, comparable to the wild-type strain. These results indicate that all lactose-oxidizing enzymes in P. taetrolens are PQQ-dependent.
Graphical Abstract






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alonso S, Rendueles M, Díaz M (2011) Efficient lactobionic acid production from whey by Pseudomonas taetrolens under pH-shift conditions. Biores Technol 102:9730–9736
Alonso S, Rendueles M, Diaz M (2013a) Feeding strategies for enhanced lactobionic acid production from whey by Pseudomonas taetrolens. Bioresour Technol 134:134–142
Alonso S, Rendueles M, Díaz M (2013b) Bio-production of lactobionic acid: current status, applications and future prospects. Biotechnol Adv 31:1275–1291
Cardoso T, Marques C, Dagostin JLA, Masson ML (2019) Lactobionic acid as a potential food ingredient: recent studies and applications. J Food Sci 84:1672–1681
Goderska K, Szwengiel A, Czarnecki Z (2014) The utilization of Pseudomonas taetrolens to produce lactobionic acid. Appl Biochem Biotechnol 173:2189–2197
Keen NT, Tamaki S, Kobayashi D, Trollinger D (1988) Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191–197
Kim J-H, Jang Y-A, Seong S-B, Jang SA, Hong SH, Song JK, Eom GT, (2020) High-level production and high-yield recovery of lactobionic acid by the control of pH and temperature in fermentation of Pseudomonas taetrolens. Bioprocess Biosys Eng 43:937–944
Kiryu T, Kiso T, Koma D, Tanaka S, Murakami H (2019) Identifying membrane-bound quinoprotein glucose dehydrogenase from acetic acid bacteria that produce lactobionic and cellobionic acids. Biosci Biotechnol Biochem 83:1171–1179
Kuusisto J, Tokarev AV, Murzina EV, Roslund MU, Mikkola J-P, Murzin DY, Salmi T (2007) From renewable raw materials to high value-added fine chemicals—Catalytic hydrogenation and oxidation of d-lactose. Catal Today 121:92–99
Matsushita K, Arents JC, Bader R, Yamada M, Adachi O, Postma PW (1997) Escherichia coli is unable to produce pyrroloquinoline quinone (PQQ). Microbiology 143:3149–3156
Murzina EV, Tokarev AV, Kordás K, Karhu H, Mikkola J-P, Murzin DY (2008) d-Lactose oxidation over gold catalysts. Catal Today 131:385–392
Nishizuka Y, Hayaishi O (1962) Enzymic formation of lactobionic acid from lactose. J Biol Chem 237:2721–2728
Nishizuka Y, Kuno S, Hayaishi O (1960) Lactose dehydrogenase, a new flavoprotein. J Biol Chem 235:Pc13-14
Oh Y-R, Eom GT (2021) Identification of a lactose-oxidizing enzyme in Escherichia coli and improvement of lactobionic acid production by recombinant expression of a quinoprotein glucose dehydrogenase from Pseudomonas taetrolens. Enzyme Microb Technol 148:109828
Oh Y-R, Eom GT (2022a) Efficient isolation of new lactobionic acid-producing microorganisms from environmental samples by colloidal calcium carbonate agar plate-based screening. Bioprocess Biosyst Eng 45:599–604
Oh Y-R, Eom GT (2022b) Efficient production of cellobionic acid from cellobiose by genetically modified Pseudomonas taetrolens. Biochem Eng J 178:108282
Oh YR, Jang YA, Lee SS, Kim JH, Hong SH, Han JJ, Eom GT (2020b) Enhancement of lactobionic acid productivity by homologous expression of quinoprotein glucose dehydrogenase in Pseudomonas taetrolens. J Agric Food Chem 68:12336–12344
Oh YR, Jang YA, Hong SH, Eom GT (2020a) Purification and characterization of a Malate: Quinone Oxidoreductase from Pseudomonas taetrolens capable of producing valuable lactobionic acid. J Agric Food Chem 68(44):13770−13778
Sarenkova I, Ciprovica I, (2018) The current status and future perspectives of lactobionic acid production : a review. 233–239
Funding
This work was supported in part by the R&D program of MOTIE/KEIT (20018375) and the R&D program of KRICT (KS2342-10, BSF23-515).
Author information
Authors and Affiliations
Contributions
SSL: investigation, validation, data curation, and writing—original draft. Y-RO: investigation, validation, data curation, and writing—original draft. Y-AJ: investigation, validation, data curation, and writing—original draft. SYH: investigation, data curation, and methodology. GTE: conceptualization, project administration, supervision, writing—original draft, writing—review and editing, funding acquisition, and resources.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work did not involve the direct study of humans. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The authors agree to publication in the International Microbiology.
The authors confirm that the work described has not been published before, that it is not under consideration for publication elsewhere, and that its publication has been approved by all co-authors.
Competing interests
The authors declare no financial or commercial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S.S., Oh, YR., Jang, YA. et al. All lactose-oxidizing enzymes of Pseudomonas taetrolens, a highly efficient lactobionic acid-producing microorganism, are pyrroloquinoline quinone-dependent enzymes. Int Microbiol (2024). https://doi.org/10.1007/s10123-023-00477-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-023-00477-4