Abstract
Gastric cancer (GC) is a common malignancy that presents challenges in patient care worldwide. The mismatch repair (MMR) system is a highly conserved DNA repair mechanism that protects genome integrity during replication. Deficient MMR (dMMR) results in an increased accumulation of genetic errors in microsatellite sequences, leading to the development of a microsatellite instability-high (MSI-H) phenotype. Most MSI-H/dMMR GCs arise sporadically, mainly due to MutL homolog 1 (MLH1) epigenetic silencing. Unlike microsatellite-stable (MSS)/proficient MMR (pMMR) GCs, MSI-H/dMMR GCs are relatively rare and represent a distinct subtype with genomic instability, a high somatic mutational burden, favorable immunogenicity, different responses to treatment, and prognosis. dMMR/MSI-H status is a robust predictive biomarker for treatment with immune checkpoint inhibitors (ICIs) due to high neoantigen load, prominent tumor-infiltrating lymphocytes, and programmed cell death ligand 1 (PD-L1) overexpression. However, a subset of MSI-H/dMMR GC patients does not benefit from immunotherapy, highlighting the need for further research into predictive biomarkers and resistance mechanisms. This review provides a comprehensive overview of the clinical, molecular, immunogenic, and therapeutic aspects of MSI-H/dMMR GC, with a focus on the impact of ICIs in immunotherapy and their potential as neoadjuvant therapies. Understanding the complexity and diversity of the molecular and immunological profiles of MSI-H/dMMR GC will drive the development of more effective therapeutic strategies and molecular targets for future precision medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer-related deaths worldwide [1]. Based on comprehensive genomic analyses, GC has been demonstrated to be a heterogeneous disease composed of different subtypes, each with peculiar molecular aspects and specific clinical behavior. GC can be categorized into four subtypes according to The Cancer Genome Atlas (TCGA) molecular classification: microsatellite instability (MSI), chromosomal instability (CIN), Epstein–Barr virus (EBV)-positive, and genomically stable (GS) tumors [2]. Similarly, the Asian Cancer Research Group (ACRG) proposed the MSI subtype as one of four molecular subtypes with distinct molecular profiles and clinical outcomes [3]. The molecular classification of GC has paved the way for personalized therapies, among which the MSI subtype has gained significant attention. Microsatellites (MSs) are widespread, short, and repetitive DNA sequences throughout the human genome that are prone to DNA replication errors [4]. As the DNA mismatch repair (MMR) system plays a key role in recognizing and correcting these errors, the genetic and epigenetic inactivation of MMR genes leads to a deficient MMR (dMMR) system, resulting in a MSI-high (MSI-H) phenotype with genomic instability and a high tumor mutation burden (TMB) [5, 6]. Thus, considerable research effort has been invested in characterizing the genomic landscape of MSI-H/dMMR GC and identifying potential therapeutic targets for precision medicine.
The discovery of immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) and programmed cell death ligand 1 (PD-L1) has led to a dramatic paradigm shift for cancer treatment, and MSI-H/dMMR is vulnerable to ICIs due to high immunogenicity and heavy infiltration of immune cells. Several pivotal trials have demonstrated that MSI-H/dMMR is significantly correlated with a response to ICIs across various types of tumor [7,8,9], indicating MSI-H/dMMR as an agnostic predictive biomarker for the efficacy of ICIs. In MSI-H/dMMR GC, treatment with ICI has shown promising and durable clinical responses, but a subset of patients still harbor intrinsic resistance [8,9,10,11,12,13,14,15,16,17,18,19]. The therapeutic paradigm will continue to evolve with an improved understanding of the immunological landscape in MSI-H/dMMR GC.
In this review, we summarize the biology, molecular and immunogenic landscape, and clinicopathological features, as well as the results of current chemotherapy and ICI treatment in MSI-H/dMMR GC. We also discuss potent therapeutic approaches in palliative and adjuvant settings, based on the state-of-the-art knowledge of MSI-H/dMMR GC from both basic and clinical viewpoints.
Clinicopathological and molecular features of MSI-H/dMMR gastric cancer
MSI-H/dMMR and microsatellite-stable (MSS)/proficient MMR (pMMR) GCs are distinct entities with widely differing genomic and immunogenic profiles and clinicopathologies (Fig. 1).
Clinicopathological features of MSI-H/dMMR gastric cancer
The DNA MMR system is a highly conserved DNA repair mechanism and is composed of not only MutL homolog 1 (MLH1), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6), and PMS1 homolog 2 (PMS2) but also mutL homologue 3 (MLH3), human mutS homologue 3 (MSH3), postmeiotic segregation increased 1 (PMS1), and exonuclease 1 (Exo1). In GC, mutations in MMR genes are relatively rare [20, 21], and MSI-H/dMMR phenotype is mostly developed from hypermethylation of CpG islands in the promoter region of the MLH1 gene and subsequent epigenetic silencing of MLH1 expression [2, 22,23,24,25,26,27,28]. Molecular testing for MSI status and immunohistochemistry testing for MMR status are equally valid as initial screening approaches and have shown a high degree of concordance [29, 30]. The frequency of MSI-H/dMMR has been reported to range between 2.6 and 35.3% of GC (Table 1) [2, 3, 12, 13, 19, 21, 28, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78]. The prevalence of MSI-H/dMMR is determined to be tumor stage-dependent, as it is higher in the early stage (5.6–35.3%) than in the advanced stage (2.6–11.7%) GC [2, 3, 12, 19, 58, 71,72,73]. Lynch syndrome (LS), caused by germline inactivating mutations in the MMR genes, had a substantial risk for GC [21, 79]. Considering the limited association of family history with the MSI-H/dMMR phenotype [24, 59] and LS [79] and recognizing the implications for cancer surveillance and prevention in affected families, the National Comprehensive Cancer Network (NCCN) clinical practice guideline recommends universal testing for MSI or MMR for all newly diagnosed GC patients [80].
MSI-H/dMMR GC exhibits distinct clinicopathological entities compared to MSS/pMMR GC (Table 1) [2, 3, 10, 12, 13, 16, 19,20,21,22, 28, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73, 75,76,77,78, 81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]. As loss of MMR function contributes to the development of tumor [20, 100], MSI-H/dMMR tumors share similar oncogenesis pathways and clinicopathological outcomes. In fact, MSI-H/dMMR GC typically exhibits predominant histopathological features of MSI-H/dMMR tumors, such as intestinal type, mucinous component, and highly pleomorphic tumor cells organized in papillary or solid-type poorly differentiated structures with prominent tumor-infiltrating lymphocytes (TILs) [20, 36, 37, 49, 58, 59, 67, 68, 82, 84, 85]. Clinically, MSI-H/dMMR GC is associated with female gender, shallower tumor invasion, early stages, and a lower number of lymph node metastases compared with MSS/pMMR GC, characteristics that are shared with MSI-H/dMMR colorectal cancer (CRC). [101, 102]. In contrast to MSI-H/dMMR CRC, MSI-H/dMMR GC has been associated with an older age of 65 years or more [2, 3, 28, 35, 37, 38, 41, 42, 47, 58, 59, 68,69,70, 81, 82]. The proportion of MSI-H/dMMR gradually increases with advancing age, accounting for 35–48% in GC patients over the age of 85 years [82, 103]. MSI-H/dMMR CRC predominantly affects the proximal colon [102], whereas MSI-H/dMMR GC is usually located in the distal stomach [3, 28, 37, 38, 41, 46, 47, 49, 54, 57, 59, 68, 70, 82].
The impact of prognosis on MSI-H/dMMR GC has mostly been evaluated by retrospective studies of resectable GC patients (Supplementary Table 1). Although conflicting results have been reported because of the limited numbers of patients, heterogeneous population with various disease stages, and different methodology for MSI/MMR detection, as well as the retrospective nature of the studies [38, 39, 58], resectable MSI-H/dMMR GC was generally associated with better prognosis than resectable MSS/pMMR GC [3, 28, 34,35,36,37, 41, 43, 44, 48,49,50,51,52,53,54,55,56,57, 59, 65,66,67, 70]. However, the prognostic value remains unclear in a setting with metastatic and recurrent unresectable GC [45, 47, 87, 104].
Molecular features of MSI-H/dMMR gastric cancer
MSI-H GC exhibits concurrent hypermethylation of multiple tumor suppressor genes, characterizing MSI-CpG island methylation phenotype (CIMP) [105]. Notably, MLH1 hypermethylation is specific to MSI-CIMP. Genetic instability caused by MSI-H/dMMR leads to the accumulation of thousands of mutations and single-nucleotide variants (SNVs) through processes such as DNA polymerase slippage and unequal crossing over [16, 106, 107], creating a hypermutator phenotype [2, 3, 23]. The MSI-H genomes are predominantly enriched for frameshift insertions or deletions (indels) but not copy number alterations [16, 106]. These genomic alterations mainly occur in MS-bearing genes and affect both coding and non-coding regions. Indels in coding genes result in frameshift mutations, leading to truncated proteins with impaired or no function. The progressive accumulation of diverse random mutations, followed by clonal selection, results in the emergence of a dominant clone displaying heightened aggressive behaviors. This evolutionary process leads to the development of an entire dysplastic lesion, referred to as field cancerization [108,109,110].
Developments in high-throughput genomic technologies have led to a better understanding of the molecular profiles in MSI-H/dMMR GC (Table 2) [2, 3, 16, 22, 23, 32,33,34, 44, 67, 68, 90, 92, 104, 107, 111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134]. Recurrent MSI has been frequently observed in specific gene clusters spanning 23 tumor types, including immune response, DNA-damage response (DDR), chromatin regulation, and transforming growth factor beta (TGF-β) [32]. MSI-H/dMMR GC shows frequent dysregulation of signaling pathways, including the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/phosphatase and tensin homolog (PTEN)/mammalian target of rapamycin (mTOR), the Wnt/β-catenin, mitotic network, chromatin regulation, DDR, and MMRs [2, 3, 20, 23, 67, 92, 107, 133]. Compared to MSS/pMMR GC, MSI-H/dMMR GC displays a higher frequency of mutations of TGF-β receptor 2 (TGFBR2), activin A receptor type 2A (ACVR2A), AT-rich interaction domain 1A (ARID1A), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), insulin-like growth factor 2 receptor (IGF2R), BCL2 associated X, apoptosis regulator (BAX), histone modifying factor, lysine methyltransferase 2C (KMT2C), lysine methyltransferase 2D (KMT2D), ring finger protein 43 (RNF43), PR/SET Domain 2 (PRDM2), and E2F transcription factor 4 (E2F4) genes but a lower incidence of TP53 mutations [2, 3, 22, 34, 68, 90, 92, 107, 111, 117, 125, 135]. Of note, an analysis of 5,930 cancer exomes from the TCGA database identified MSI loci with high instability in specific tumor types, leading to tumor-specific instability signatures [33]. For instance, BAX, PRDM2, mediator complex subunit 1 (MED1), and KMT2C are enriched in MSI-H/dMMR GC [32, 125]. In contrast, the B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutation and neurotrophic tyrosine receptor kinase (NTRK) gene fusion have been identified as prevalent in MSI-H/dMMR CRC, whereas these alterations are not enriched in MSI-H/dMMR GC [2, 32, 136]. Thus, impairment of the MMR system drives oncogenic deregulation in both cancer-specific and MSI-specific ways. Importantly, parallel evolution of subclonal driver mutations occurs in RAS, PIK3CA, switch/sucrose nonfermentable (SWI/SNF)-complex genes, and immune evasion regulators. The MSI hypermutator phenotype remains active during cancer progression, generating more subclonal mutations and, consequently, extreme intratumoral heterogeneity.
Immunological features of MSI-H/dMMR gastric cancer
The heightened occurrence of nonsynonymous SNVs and frameshift mutations generates numerous immunogenic neoantigens on the major histocompatibility complex (MHC) class I molecules on tumor cells and on MHC class I and II on antigen-presenting cells, thereby priming T cells to identify them as non-self and recruiting T cells within the tumor as TILs [11, 137, 138]. In fact, MSI-H GCs are characterized by prominent TILs [16, 36, 86,87,88,89,90,91,92,93,94,95,96,97]. In addition, MSI-H/dMMR GCs exhibit TILs containing abundant M1 macrophages and natural killer (NK) cells [139,140,141], along with activated CD8+ cytotoxic T-lymphocytes and T helper type 1 cells characterized by interferon-gamma (IFN-γ) response [16, 86, 89, 90, 92, 93, 95]. A transcriptome and RNA sequencing (RNA-seq) analysis revealed enrichment of pathways related to immune cell signaling, cytotoxicity, NK cell function, and antigen processing in MSI-H GC [2, 86]. Consequently, in the early stage, activated immunosurveillance may contribute to favorable prognostic outcomes in patients with MSI-H/dMMR GC compared to those with MSS/pMMR GC [3, 91, 93]. However, tumor elimination or immune surveillance is not always efficient. Cancer immunoediting is profoundly influenced by immune selective pressure stemming from the high immunogenicity of MSI-H/dMMR tumors, ultimately leading to immune escape [142]. Of note, MSI-H/dMMR tumors also stimulate the expression of inhibitory immune checkpoint molecules, including PD-1 and PD-L1. The PD-L1/PD-1 signaling axis creates an immune-evasive state in the tumor microenvironment (TME) [6]. In GC, PD-L1 is more frequently overexpressed on both tumor cells and immune cells in MSI-H/dMMR tumors compared to MSS/pMMR tumors [2, 10, 86, 88,89,90, 94, 96, 98, 99]. Based on their PD-L1 expression and the number of TILs, tumors can be classified into four subtypes according to the tumor microenvironment immune type (TMIT), which predicts suitable candidates for immunotherapy [143]. An RNA analysis of 414 GC samples in the pan-cancer database of TCGA revealed that approximately 70% of MSI-H/dMMR GCs exhibited TMIT type I, characterized by high PD-L1 and CD8A expression [95], driving adaptive immune resistance. Therefore, PD-1/PD-L1 blockade may reverse the immune-evasive state into an antitumor response state, providing a rationale for treating MSI-H/dMMR GC patients with ICIs targeting PD-1/PD-L1.
Treatment of patients with MSI-H/dMMR gastric cancer
In this section, the clinical efficacy of chemotherapy agents for patients with MSI-H/dMMR GC is described (Fig. 1).
Cytotoxic chemotherapy
As the MMR system not only repairs DNA replication errors but also activates signaling pathways that trigger apoptosis in response to DNA damage, impairment of the MMR system may be a relevant mechanism of resistance to a variety of cytotoxic agents, including 5-fluorouracil (5-FU) and cisplatin [83, 144,145,146,147,148,149]. In CRC, MSI-H/dMMR tumors are associated with a favorable prognosis and lack of efficacy of adjuvant fluoropyrimidine monotherapy [62, 101, 150], as well as little benefit from neoadjuvant fluoropyrimidine-based chemotherapy [151], suggesting consideration of MSI/MMR status in treatment decision-making. The impact of cytotoxic chemotherapy on perioperative settings, including adjuvant chemotherapy, for MSI-H/dMMR GC patients has been evaluated in several exploratory analyses and retrospective studies (Supplementary Table 1).
Patients with MSI-H/dMMR GC might have limited benefits from neoadjuvant chemotherapy [30, 60,61,62, 65, 70]. In addition, MSI-H/dMMR tumors exhibited low pathological tumor response to neoadjuvant chemotherapy [30, 37, 61, 62, 64, 152, 153]. Similarly, many retrospective studies have reported no treatment benefit from 5-FU-based adjuvant chemotherapy [38,39,40, 43, 46, 47, 50,51,52, 56, 154], despite some conflicting results [42, 44, 54, 55]. In terms of oxaliplatin (L-OHP) among platinum compounds, the addition of L-OHP to adjuvant treatment with 5-FU demonstrated prolonged survival compared to those with 5-FU alone for patients with stage III MSI-H/dMMR CRC [155]. In GC, a post hoc analysis was performed according to the MSI status in a phase III CLASSIC trial that demonstrated the survival benefit of adjuvant CAPOX (capecitabine plus L-OHP) chemotherapy over surgery alone for patients with stage II and III GC [50]. Adjuvant CAPOX chemotherapy failed to demonstrate an improvement in disease-free survival (DFS) for patients with MSI-H, in contrast to those with MSS. In a meta-analysis of perioperative chemotherapy using only randomized individual patient data from four-phase III resectable GC trials [52], chemotherapy plus surgery showed significantly prolonged survival compared to surgery alone in patients with MSS but not in those with MSI-H. It remains unclear whether taxanes, such as docetaxel and paclitaxel, are effective against MSI-H/dMMR GC tumors [74, 156, 157].
Collectively, previous studies have challenged the clinical benefits of perioperative chemotherapy in MSI-H/dMMR patients due to their favorable prognosis and the limited efficacy of chemotherapy, raising the possibility of avoiding unnecessary chemotherapy for patients with resectable MSI-H/dMMR GC, especially for older patients with early-stage disease. However, the routine clinical use of MSI/MMR status in therapeutic decision-making for perioperative chemotherapy, including adjuvant chemotherapy, is still debated due to limited and retrospective data [158,159,160], emphasizing the need for large prospective trials based on MSI status. In a palliative setting, exploratory analyses of clinical trials showed that chemotherapy, including 5-FU plus platinum or paclitaxel, has equivalent outcomes in patients with MSI-H/dMMR or MSS/pMMR GC (Supplementary Table 1).
Immune therapy
ICIs targeting PD-1 have dramatically changed therapeutic paradigms due to the durable clinical response in GC [8,9,10,11,12,13,14,15,16,17,18,19, 76, 161]. However, the efficacy of ICI monotherapy is limited to certain patient populations [80, 158, 162,163,164]. Building on the clinical benefits observed with anti-PD-1 antibody (Ab) pembrolizumab in MSI-H/dMMR tumors across various organ sites [7,8,9, 11], the Food and Drug Administration granted the first tumor-agnostic approval for pembrolizumab in May 2017 for MSI-H/dMMR tumors. The clinical efficacy of PD-1/PD-L1 Ab for patients with MSI-H/dMMR GC in pivotal trials is summarized in Table 3 [8, 9, 11,12,13,14,15,16,17,18,19, 71, 76, 165,166,167].
Palliative immune therapy
Although ICI monotherapy demonstrated no improvement in overall survival (OS) compared to standard chemotherapy in the overall population in the phase III KEYNOTE-062 and KEYNOTE-061 trials [165, 167], among patients with MSI-H/dMMR GC, ICI monotherapy consistently showed a superior survival curve compared to chemotherapy from the beginning of treatment, accompanied by higher overall response rate (ORR) and prolonged progression-free survival (PFS) [12]. The positive effects of PD-1/PD-L1 Ab for patients with MSI-H/dMMR GC have been confirmed in several systematic reviews and meta-analyses [72, 73, 168,169,170,171]. Thus, the MSI-H/dMMR status serves as a promising molecular hallmark, indicating potential sensitivity to ICI treatment, with an ORR ranging from 29 to 60% and a disease control rate (DCR) ranging from 48 to 89% (Table 3) [8, 9, 11,12,13,14,15,16,17,18,19, 71, 76, 165,166,167]. Recently, the clinical benefits of anti-PD-1 Ab in combination with first-line chemotherapy for patients with the human epidermal growth factor receptor 2 (HER2)-negative GC were demonstrated in pivotal phase III trials [71, 76, 161, 172,173,174]. A systematic review and meta-analysis were conducted to evaluate the treatment efficacy for 3355 GC patients using five randomized phase III trials of the addition of anti-PD-1 Ab to first-line cytotoxic chemotherapy [168]. The estimated HR of OS in the combination of anti-PD-1 Ab and chemotherapy versus chemotherapy alone was significantly improved in MSI-H patients (HR, 0.38; 95% CI 0.20–0.70) compared to MSS patients (HR, 0.78; 95% CI 0.70–0.87).
For MSI-H/dMMR tumors, the optimal treatment regimen, whether ICI monotherapy or ICI combined with chemotherapy, remains uncertain. Chemotherapy has been demonstrated to induce immunogenic cell death in tumor cells, triggering recognition by dendritic cells (DCs) and the activation of CD8+ T cells [175]. Consequently, combining ICI with chemotherapy may offer a promising approach to overcoming primary resistance to immunotherapy. In the phase III KEYNOTE-062 trial, among patients with MSI-H tumors and a PD-L1 combined positive score of 1 or greater, pembrolizumab monotherapy demonstrated a trend toward a more favorable OS compared to chemotherapy alone (HR, 0.29; 95% CI 0.11–0.81), while pembrolizumab plus chemotherapy showed a slightly lower efficacy (HR, 0.37; 95% CI 0.14–0.97) [12, 165]. Conversely, pembrolizumab plus chemotherapy showed superior ORR and PFS compared to pembrolizumab monotherapy, with an ORR of 64.7% and estimated HR for PFS of 0.45 (95% CI 0.18–1.11) versus an ORR of 57.1% and HR for PFS of 0.72 (95% CI 0.31–1.68), respectively. Although this trial was not intended to directly compare pembrolizumab alone and pembrolizumab in combination with chemotherapy, the results suggest a potential benefit for MSI-H/dMMR patients when receiving a combination of chemotherapy on multiple occasions to induce an initial response. However, prolonged administration of chemotherapy may not provide additional benefits. Based on the hypothesis that MSI-H/dMMR patients might benefit from a short course of chemotherapy, an ongoing phase II AuspiCiOus proof-of-principle trial aims to assess the treatment efficacy of a sequential method involving two cycles of CAPOX chemotherapy, followed by monotherapy with the anti-PD-1 Ab retifanlimab (NCT05177133).
A relevant proportion of MSI-H/dMMR GC patients undergoing anti-PD-1/PD-L1 monotherapy still exhibit intrinsic resistance, with a progressive disease rate of 20–50% in clinical trials (Table 3), indicating that MSI-H/dMMR GCs still display substantial heterogeneity from an immunological viewpoint. Molecular analyses of MSI-H/dMMR GC underscore alterations in genes that regulate the antigen-presenting machinery, IFN-γ signaling, Wnt/β-catenin pathway, TGF-β pathway, and PI3K pathway, contributing to resistance to ICI treatment (Table 2) [2, 16, 92, 131, 138, 176,177,178,179,180]. In addition, a comprehensive assessment of various components within the TME will be crucial for a more accurate prediction of the success or failure of ICI treatment in patients with MSI-H GC [16, 181, 182]. Thus, elucidating the determinant mechanisms of immunotherapy sensitivity and resistance would pave the way for the development of new treatment strategies.
Potent therapeutic strategies in MSI-H/dMMR gastric cancer
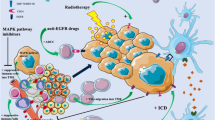
In this section, we summarize the findings from previous studies and explore potential therapeutic strategies for patients with MSI-H/dMMR GC. These strategies include a dual immune checkpoint blockade, combining PD-1/PD-L1 Abs with molecular targeted agents, and targeting vulnerabilities with selective molecule inhibition (Fig. 2).
Summary of potent therapeutic strategies for MSI-H/dMMR GC. In the perioperative setting, neoadjuvant or perioperative ICI therapy has already become one of the options for patients with MSI-H/dMMR GC in the NCCN guidelines. Besides monotherapy with ICIs, combining anti-PD-1/PD-L1 Ab with anti-CTLA-4 Ab or other ICIs, including TIGIT, TIM3, and LAG-3 inhibitors, as well as an OX40 agonist Ab, are also considered. In the metastatic setting, potent strategies include dual immune checkpoint blockade, combining anti-PD-1/PD-L1 Ab with molecular targeted agents, and targeting vulnerabilities with selective molecule inhibition. A dual immune checkpoint blockade include combination of anti-PD-1/PD-L1 Ab with anti-CTLA-4 Ab or other ICIs. Mutations of PRKDC, KMT2D, and KMT2C gene mutations potentially serve as predictive biomarkers for immunotherapy response. Effective partners for ICIs include angiogenesis inhibitors such as apatinib and lenvatinib, Nedd8-activating enzyme inhibitors, and DDR inhibitors for ARID1A deficiency. Promising therapeutic targets for MSI-H/dMMR tumors include inhibitors of WRN, TGF-β, PI3K/Akt, Wnt/β-catenin, AURK, BET for BAZ1B-dependent tumors, RARP for KMT2C and KMT2D mutant tumors, and RAS for RAS mutant tumors. ARID1A-deficient tumors may benefit from targeting ATR checkpoint activity, the non-catalytic role of EZH2, the PI3K/Akt pathway, PARP, and HDAC inhibitors
Immune therapy in a palliative setting
Several treatment strategies have been explored to transform immunologically “cold” tumors with poor immune activation into “hot” tumors with robust immune infiltration. The evolving landscape of immunotherapy in MSI-H/dMMR GC highlights the importance of combination approaches targeting multiple immune checkpoints and pathways to overcome resistance mechanisms. Combination therapies of anti-PD-1/PD-L1 Ab with other immune-modulating treatments, including other ICIs, angiogenetic inhibitors, and molecular targeted agents, present a promising avenue for treating MSI-H/dMMR GC by enhancing treatment efficacy through potential synergistic effects (Tables 4 and 5 and Fig. 2).
Dual inhibition of immune checkpoint molecules
The PD-1/PD-L1 interaction is just one of several immune checkpoint pathways regulating T cell activation in the TME. Other molecules, such as anti-cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), T cell immunoreceptor with Ig and ITIM domains (TIGIT), T cell immunoglobulin mucin receptor 3 (TIM3), and lymphocyte activation gene 3 protein (LAG-3), are also overexpressed in various immune cells and act as inhibitory immune checkpoint modulators in GC (Table 4) [130, 183]. These inhibitory immune checkpoints may induce tumors to lose immunogenicity, contributing to reduced sensitivity to immunotherapy [6, 137, 184].
The most promising strategy is the dual blockade of PD-1 and CTLA-4. The phase I/II CheckMate-032 trial assessed the efficacy and safety of anti-PD-1 Ab nivolumab monotherapy or two different schedules of nivolumab plus anti-CTLA-4 Ab ipilimumab in patients with chemotherapy-refractory GC [14]. Patients were randomly assigned to one of the following treatment groups: nivolumab at 3 mg/kg (NIVO3); nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg (NIVO1 + IPI3) every 3 weeks for four cycles; or nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg (NIVO3 + IPI1) every 3 weeks for four cycles. All combination regimens were followed by NIVO3 every 2 weeks until disease progression or unacceptable toxicity. The NIVO1 + IPI3 group showed the highest ORR and 12-month PFS rate among the three groups, despite having the highest frequency of treatment-related adverse events. In a subset of MSI-H GC patients, treatment with nivolumab plus ipilimumab showed comparable outcomes in the ORR and 18-month PFS rates in the NIVO1 + IPI3 and NIVO3 + IPI1 groups. This approach demonstrated a tendency toward improved ORR (50% vs. 29%) and 18-month PFS rates (50% vs. 29%) compared to nivolumab monotherapy. In a phase III CheckMate 649 trial, the combination of nivolumab plus ipilimumab, as well as nivolumab plus chemotherapy, versus chemotherapy alone was evaluated in the first-line setting for GC [71]. Based on the results of the CheckMate-032 trial, the NIVO1 + IPI3 schedule was adopted. A limited number of patients had the MSI-H phenotype, with 11 patients for nivolumab plus ipilimumab and 10 patients for chemotherapy alone. Although the treatment group with nivolumab plus ipilimumab was discontinued early due to unacceptable toxicities, nivolumab plus ipilimumab showed a higher ORR (70% vs. 57%) and a longer median OS (HR, 0.28; 95% CI 0.08–0.92) compared with chemotherapy alone in patients with MSI-H GC. The group with nivolumab plus ipilimumab also demonstrated a more favorable ORR (70% vs. 55%) and an estimated HR for OS (0.28 vs. 0.38) than the group with nivolumab plus chemotherapy in a small subset of patients with 29 patients with MSI-H GC. In a single-arm phase II NO LIMIT trial of first-line nivolumab plus ipilimumab for MSI-H GC [185], low-dose ipilimumab at 1 mg/kg was chosen to reduce toxicity, considering the higher toxicity of the NIVO1 + IPI3 schedule in the CheckMate-032 trial. The ORR was 62.1%, with a clinical complete response (CR) rate of 10.3% and a DCR of 79.3%. The median PFS was 13.8 months, and the 12-month OS rate was 80%, suggesting a potential chemotherapy-free option for MSI-H GC patients. However, treatment-related adverse events led to discontinuation in 44.8% of the patients, indicating that further development of this regimen may require adjustments for improved feasibility. A phase III ONO-4538-113 trial (NCT05144854) to compare the efficacy and safety of nivolumab plus ipilimumab in combination with chemotherapy versus chemotherapy alone as a first-line treatment for patients with GC is currently underway.
Targeting various immune checkpoints, such as TIGIT, TIM3, LAG-3, and OX40, as well as CTLA-4, has shown promising results in preclinical and clinical studies in various types of tumors, suggesting their potential as therapeutic strategies in cancer treatment (Table 4). The efficacy of dual blockade targeting TIGIT and PD-1 is being assessed in the phase III STAR-221 trial (NCT05568095) in the first-line setting for GC, as well as in a cohort of metastatic MSI-H tumors from the phase II basket TIRACAN trial (NCT05483400) (Table 5). The efficacy of OX40 agonist Ab is currently under investigation in a phase I/II trials in certain solid tumors, including MSI-H/dMMR tumors (NCT04198766 and NCT03894618).
Combination with angiogenesis‑ or other molecular‑targeted agents
Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling pathway induces immunosuppressive effects via the downregulation of MHC expression, the activation of inhibitory immune checkpoint molecules, and the inhibition of TILs and DC differentiation [6, 186]. The most compatible partners of ICIs have been found to be anti-angiogenic inhibitors and platinum chemotherapy in a cross-sectional study of 98 clinical trials that included 24,915 patients [187], supporting combination treatment utilizing an anti-angiogenic inhibitor with ICIs (Table 4). Currently, several trials evaluating the efficacy of combining ICIs with anti-angiogenic inhibitors are ongoing in GC (NCT03407976 and NCT04662710) (Table 5). The understanding of angiogenesis in the TME may contribute to overcoming primary resistance to ICI in MSI-H/dMMR GC.
The PI3K/PTEN/v-akt murine thymoma viral oncogene homolog (Akt)/mTOR pathway is more frequently dysregulated in MSI-H GC than MSS GC (Table 2), and the activated signaling potentially predicts primary resistance to ICIs in MSI-H/dMMR GC [92, 180]. Conversely, MSI-H/dMMR GC with mutated genes in the PI3K pathway showed sensitivity to inhibitors of the PI3K pathway [92]. Simultaneous inhibition of the PI3K pathway may overcome resistance to ICIs as an immunotherapeutic adjunct in populations with activated PI3K pathways. As the H1047R mutation in exon 20 of PIK3CA is a common alteration in MSI-H/dMMR GC [3], the selective PI3Kα H1047R inhibitor, such as LOXO-783, may have a therapeutic effect [188]. Ipatasertib is a highly selective ATP-competitive pan-Akt inhibitor targeting phosphorylated Akt1-3. In a phase II trial (NCT04739202), patients with GC positive for EBV or MSI-H will receive treatment with atezolizumab and ipatasertib (Table 5).
A deficient MMR system causes cells to accumulate shared immunogenic frameshift peptide neoantigens [189]. In a phase I/IIa trial evaluating the safety and immunogenicity of a frameshift peptide neoantigen-based vaccine in patients with MSI/dMMR CRC, this approach showed a promising novel strategy for the treatment and prevention of dMMR tumors [190]. Molecules in the TGF-β, Wnt/β-catenin, DDR, and NEDD8 ubiquitin-like modifier (Nedd8)-mediated degradation pathways are also reported as potent therapeutic targets (Table 4). An in-depth understanding of the functional roles and molecular mechanisms of MSI-H/dMMR GC is crucial for developing targeted therapies.
Immune therapy in a perioperative setting
Several trials have reported the promising treatment efficacy of neoadjuvant ICI in lung cancer, melanoma, bladder cancer, and CRC [191,192,193,194,195], indicating that immunotherapy may be highly effective in patients with early-stage cancer. Furthermore, neoadjuvant anti-PD-1 Ab dostarlimab monotherapy has demonstrated highly impressive results in that all 12 patients had CR and consequently avoided chemoradiotherapy and surgery in locally advanced dMMR rectal cancer [196]. The encouraging findings raise the hypothesis that the clinical efficacy of ICI treatment in early-stage MSI-H/dMMR tumors, prior to the emergence of immune evasion mechanisms facilitating metastatic dissemination, may surpass that in metastatic stages, prompting an exploration of ICI treatment in resectable MSI-H/dMMR GC (Table 5).
The integration of ICI into the perioperative chemotherapy has not been established across all populations of GC [77, 197]. However, in a meta-analysis of seven prospective phase I/II trials on ICI-based neoadjuvant therapy, 57 patients with MSI-H/dMMR GC had higher rates of pathological complete response (pCR) and major pathologic responses (MPR) compared to 244 patients with MSS/pMMR GC [198]. The potential benefits of ICI plus chemotherapy for MSI-H/dMMR GC have been observed in exploratory analyses of the phase III KEYNOTE-585 [77] and ongoing MATTERHORN (NCT04592913) [78] trials, as well as in recent phase II trials [74, 199,200,201]. Several trials are evaluating the efficacy of combining ICI with perioperative chemotherapy (NCT03421288, NCT04139135, NCT04592913, NCT04744649) (Table 5). Subgroup data derived from the MSI/MMR status in these trials would enhance the hypothesis that perioperative ICI treatment is beneficial for MSI-H/dMMR GC patients.
It is crucial to evaluate the need to incorporate cytotoxic agents into immunotherapy for an optimal treatment strategy. A phase II GERCOR NEONIPIGA trial evaluated the pathological response rate and safety of neoadjuvant nivolumab plus low-dose ipilimumab at 1 mg/kg for six cycles, followed by adjuvant nivolumab for 9 months in patients with resectable MSI-H/dMMR GC [202]. Of the 32 enrolled patients, 29 underwent curative surgery, with a pCR rate of 58.6%. The rate of pathological complete regression in the primary tumor for patients with MSI-H/dMMR was 66% in this trial using neoadjuvant ICI alone, comparable to the rate of 66% for patients with MSI-H/dMMR in the DANTE/IKF-s633 trial using ICI plus chemotherapy [74], prompting inquiries into the potential benefits of perioperative cytotoxic agents. At a median follow-up of 12 months, 30 (93.7%) patients remained alive without disease progression. It is noteworthy that three patients who did not undergo surgery achieved clinical CR and remained event-free. A phase II INFINITY trial (NCT04817826) is investigating dual ICI blockade using durvalumab plus anti-CTLA-4 Ab tremelimumab in the neoadjuvant (cohort 1) and potentially definitive (cohort 2) treatment for resectable MSI-H/dMMR GC [203]. In a cohort 1, 15 patients received a 12-week treatment followed by surgery, achieving a pCR rate of 60%. No disease relapses were observed in all patients with pCR. Non-operative management following the same regimen is being explored with cohort 2. Other ongoing phase II trials of perioperative treatment with ICI alone include NCT04795661, NCT03257163, NCT05994456, and NCT04556253 (Table 5). A direct comparison of perioperative ICI plus chemotherapy against perioperative ICI monotherapy is being evaluated in a randomized phase II ECOG-ACRIN trial (NCT05836584). These trials will provide proof-of-concept data for potentially omitting chemotherapy or surgery in selected patients after neoadjuvant immunotherapy.
Several preclinical studies have shown that neoadjuvant PD-1/PD-L1 blockade disrupts immunodominance and facilitates the early establishment of immunological memory following primary tumor resection, a phenomenon not observed in the adjuvant setting [204, 205]. This phenomenon contributes to the eradication of minimal residual disease and micro-metastases. Consequently, the efficacy of ICIs as an adjuvant therapy after surgery in improving outcomes for MSI-H/dMMR GC patients remains uncertain. Valuable insights are expected from the exploratory analysis of data from ICI-containing adjuvant trials, such as the phase III ATTRACTION-5 trial [197] and the phase III CheckMate-577 trial [206]. Currently, several phase II trials have investigated the treatment efficacy of adjuvant ICI (NCT05769725 and NCT05468138) (Table 5).
Although the limited sample size and short follow-up period hindered conclusive evidence, a growing number of impressive outcomes suggest a potential paradigm shift in approach to neoadjuvant immunotherapy or non-operative strategies for early-stage MSI-H/dMMR GC, emphasizing the importance of future dedicated clinical trials. Further research is required to determine the optimal regimen and duration for perioperative immunotherapy.
Potent molecular‑targeted therapies
Therapeutic vulnerability has garnered considerable attention as a new hope for patients with MSI-H/dMMR GC (Table 4 and Fig. 2). DNA repair processes represent attractive synthetic lethal targets in MSI-H/dMMR tumors due to impaired DNA repair pathways, leading to a reliance on specific repair proteins. For example, the downregulation of KMT2C in bladder cancer leads to changes in the epigenetic status and expression of DDR and DNA repair genes, particularly affecting homologous recombination-mediated DNA double-strand break (DSB) repair. Thus, cancers with low KMT2C expression rely heavily on poly (ADP-ribose) polymerase (PARP) for DNA repair, and treatment with the PARP inhibitor leads to synthetic lethality [207]. In MSI-H/dMMR GC, KMT2C and KMT2D mutations are linked to DNA repair, making them potential targets for treatment with PARP inhibitors. The SWI/SNF complex is frequently mutated in MSI-H/dMMR GC, and therapeutic agents targeting SWI/SNF are emerging [208]. SWI/SNF-altered cancers may be sensitive to DNA-damage repair inhibitors and ICIs [133, 208].
As the aberration of ARID1A promotes increased reliance on ATR checkpoint activity caused by topoisomerase 2A and cell cycle defects, ATR inhibitors can act as synthetic lethal therapy for ARID1A-deficient tumors, both in vitro and in vivo [209]. ARID1A-deficient cancers are dependent on the non-catalytic role of the zeste 2 polycomb repressive complex 2 subunit (EZH2), promising therapeutic utility for ARID1A-deficient tumors [210]. Several preclinical studies have reported other crucial targets that induce synthetic lethality with ARID1A deficiency, such as PARP [211], ARID1B [212], the glutamate-cysteine ligase synthetase catalytic subunit [213], histone deacetylase 6 (HDAC6) [214], and BIRC5/Survivin [215]. Thus, inhibiting molecules that create the therapeutic vulnerability of ARID1A-deficient tumor cells may be of clinical importance.
In a CRISPR/dCas9 genome-wide screening of MSH2-deficient GC cells, the bromodomain adjacent to zinc finger domain 1B (BAZ1B) was identified as a synthetic lethal partner [216]. As both MSH2 and BAZ1B play roles in regulating the transcription of cell adhesion genes, MSH2-deficient GC cells can become dependent on BAZ1B, leading to synthetic lethality through the inhibition of the bromodomain and extraterminal motif (BET). This effect has also been observed in MSI-H GC cells. MSI-H/dMMR GCs show increased expression of mitotic network components, including aurora kinase (AURK) [2, 32]. AURK inhibitors were identified as potential candidate drugs for GCs with high immune activity, characterized by high TMB-H and MSI-H, based on the connectivity map database and gene set enrichment analysis [217].
Werner syndrome protein (WRN) is a RecQ enzyme that plays a crucial role in genome maintenance. Inhibition of WRN leads to DSBs, which selectively induce cell cycle arrest and apoptosis in MSI-H/dMMR tumors, including GC, due to their reliance on WRN’s helicase activity, unlike MSS/pMMR tumors [218]. Therefore, WRN represents a potential synthetic lethal vulnerability and a promising therapeutic target for MSI-H/dMMR tumors. HRO761, an allosteric WRN inhibitor, binds to the interface of the D1 and D2 helicase domains, rendering WRN inactive. This leads to WRN protein degradation and activates the DNA-damage response, resulting in tumor growth inhibition, specifically in MSI-H cell and patient-derived xenograft models [219]. A phase I trial is currently underway to evaluate the safety, tolerability, and preliminary antitumor activity of HRO761 alone or in combination with anti-PD-1 Ab tislelizumab or irinotecan in patients with MSI-H/dMMR solid tumors (NCT05838768) (Table 5).
MSI-H/dMMR GC typically lacks targetable amplifications. Notably, patients with BRAF-mutated MSI-H CRC had favorable outcomes with BRAF-targeted inhibitors in the phase III BEACON CRC trial [220], suggesting that molecular targeted therapy holds promise for MSI-H/dMMR GC. Considering the high incidence of Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation in MSI-H/dMMR GC [2, 118, 221], the success in targeting KRAS G12C offers hope for developing allele-specific therapies for various mutant RAS alleles [222]. MSI-H/dMMR GC generally lacks targetable amplifications of HER2 but shows its frequent mutation [2]. In HER2-mutant non-small-cell lung cancer, an HER2 Ab-drug conjugate trastuzumab deruxtecan showed durable antitumor activity [223]. Loss of ARID1A leads to activation of the PI3K/Akt pathway via concurrent PIK3CA mutation and accelerated phosphorylation of Akt, and ARID1A-deficient GCs may be vulnerable to inhibitors of Akt or PIK3CA [114, 224].
The identification of a population vulnerable to specific molecular inhibition could lead to personalized molecular targeted medicine for MSI-H/dMMR GC patients (Fig. 2). In addition, assessing circulating tumor DNA (ctDNA) for intra- and inter-tumoral heterogeneity can help identify clonally altered genes in MSI-H/dMMR GC, guiding the selection of patients who may benefit from molecular targeted agents. As there is currently insufficient scientific evidence to establish these therapeutic strategies for MSI-H/MMR GC, further preclinical and clinical studies are needed for MSI-H/MMR GC.
Conclusion
MSI-H/dMMR GC is a distinct subtype characterized by specific molecular features and clinical implications. Testing for MSI or MMR status should be a standard practice to guide treatment selection in GC patients. Immunotherapy has shown promise in treating metastatic MSI-H/dMMR GC, and neoadjuvant ICIs have implications for organ-sparing strategies. However, MSI-H/dMMR GC exhibits significant heterogeneity in terms of genomic, immunologic, and clinical outcomes, and a subset of patients treated with ICI exhibits primary resistance. Future research should focus on developing biomarker-driven treatment strategies, identifying novel therapeutic targets, and exploring synergistic therapeutic partners to improve prognostic outcomes in MSI-H/dMMR GC. A deeper understanding of the biology of MSI-H/dMMR GC could reveal a population vulnerable to specific molecular inhibition, potentially leading to the establishment of personalized medicine.
Data availability
N/A.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56.
Boland CR, Thibodeau SN, Hamilton SR, et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Ooki A, Shinozaki E, Yamaguchi K. Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon. 2021;5:11–24.
Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33:929–38.
Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10.
Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58.
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7:895–902.
Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4: e180013.
Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36:2836–44.
Ott PA, Le DT, Kim JW, et al. Nivolumab (NIVO) in patients (pts) with advanced (adv) chemotherapy-refractory (CT-Rx) esophagogastric (EG) cancer according to microsatellite instability (MSI) status: checkmate 032. Ann Oncol. 2017;28:v229–30.
Kwon M, An M, Klempner SJ, et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021;11:2168–85.
Andre T, Berton D, Curigliano G, et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: results from GARNET study. J Clin Oncol. 2021;39:9–9.
Li J, Deng Y, Zhang W, et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol Oncol. 2021;14:95.
Moehler M, Dvorkin M, Boku N, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39:966–77.
Ottini L, Falchetti M, Lupi R, et al. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol. 2006;17(Suppl 7):vii97-102.
Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol. 2019;37:286–95.
Wu MS, Lee CW, Shun CT, et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000;27:403–11.
von Loga K, Woolston A, Punta M, et al. Extreme intratumour heterogeneity and driver evolution in mismatch repair deficient gastro-oesophageal cancer. Nat Commun. 2020;11:139.
Leite M, Corso G, Sousa S, et al. MSI phenotype and MMR alterations in familial and sporadic gastric cancer. Int J Cancer. 2011;128:1606–13.
Lee JH, Park SJ, Abraham SC, et al. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–54.
Hu G, Qin L, Zhang X, Ye G, Huang T. Epigenetic silencing of the MLH1 promoter in relation to the development of gastric cancer and its use as a biomarker for patients with microsatellite instability: a systematic analysis. Cell Physiol Biochem. 2018;45:148–62.
Ye P, Shi Y, Li A. Association between hMLH1 promoter methylation and risk of gastric cancer: a meta-analysis. Front Physiol. 2018;9:368.
Tran-Minh ML, Lehmann-Che J, Lambert J, et al. Prevalence and prognosis of microsatellite instability in oesogastric adenocarcinoma, NORDICAP 16–01. Clin Res Hepatol Gastroenterol. 2021;45:101691.
de Guyot D’Asnières Salins A, Tachon G, Cohen R, et al. Discordance between immunochemistry of mismatch repair proteins and molecular testing of microsatellite instability in colorectal cancer. ESMO Open. 2021;6:100120.
Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–203.
Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. https://doi.org/10.1200/PO.17.00073.
Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180.
Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–50.
Schneider BG, Bravo JC, Roa JC, et al. Microsatellite instability, prognosis and metastasis in gastric cancers from a low-risk population. Int J Cancer. 2000;89:444–52.
Beghelli S, de Manzoni G, Barbi S, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–56.
Mathiak M, Warneke VS, Behrens HM, et al. Clinicopathologic characteristics of microsatellite instable gastric carcinomas revisited: urgent need for standardization. Appl Immunohistochem Mol Morphol. 2017;25:12–24.
Biesma HD, Soeratram TTD, Sikorska K, et al. Response to neoadjuvant chemotherapy and survival in molecular subtypes of resectable gastric cancer: a post hoc analysis of the D1/D2 and CRITICS trials. Gastric Cancer. 2022;25:640–51.
An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–11.
Oh N, Kim H, Kim KM, et al. Microsatellite instability and effectiveness of adjuvant treatment in pT1N1 gastric cancer: a multicohort study. Ann Surg Oncol. 2021;28:8908–15.
Kim SY, Choi YY, An JY, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer. 2015;137:819–25.
Marrelli D, Polom K, Pascale V, et al. Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol. 2016;23:943–50.
Sohn BH, Hwang JE, Jang HJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017;23:4441–9.
Huang SC, Ng KF, Yeh TS, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145:3218–30.
Kim JW, Cho SY, Chae J, et al. Adjuvant chemotherapy in microsatellite instability-high gastric cancer. Cancer Res Treat. 2020;52:1178–87.
An JY, Choi YY, Lee J, et al. A multi-cohort study of the prognostic significance of microsatellite instability or mismatch repair status after recurrence of resectable gastric cancer. Cancer Res Treat. 2020;52:1153–61.
Tsai CY, Lin TA, Huang SC, et al. Is adjuvant chemotherapy necessary for patients with deficient mismatch repair gastric cancer?-Autophagy inhibition matches the mismatched. Oncologist. 2020;25:e1021–30.
Guan WL, Ma Y, Cui YH, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: a multicenter analysis. Front Oncol. 2021;11:712760.
Di Bartolomeo M, Morano F, Raimondi A, et al. Prognostic and predictive value of microsatellite instability, inflammatory reaction and PD-L1 in gastric cancer patients treated with either adjuvant 5-FU/LV or sequential FOLFIRI followed by cisplatin and docetaxel: a translational analysis from the ITACA-S Trial. Oncologist. 2020;25:e460–8.
Miceli R, An J, Di Bartolomeo M, et al. Prognostic impact of microsatellite instability in Asian gastric cancer patients enrolled in the ARTIST trial. Oncology. 2019;97:38–43.
Choi YY, Kim H, Yang H-K, et al. Clinical impact of microsatellite instability in patients with stage II and III gastric cancer: results from the CLASSIC trial. J Clin Oncol. 2017;35:4022–4022.
Choi YY, Kim H, Shin SJ, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post Hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019;270:309–16.
Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–400.
Choi YY, Bae JM, An JY, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110:129–35.
Nie RC, Chen GM, Yuan SQ, et al. Adjuvant chemotherapy for gastric cancer patients with mismatch repair deficiency or microsatellite instability: systematic review and meta-analysis. Ann Surg Oncol. 2022;29:2324–31.
Zhao F, Yuan X, Ren D, et al. Predicting the efficacy of 5-fluorouracil-based adjuvant chemotherapy in gastric cancer by microsatellite instability: a meta-analysis. J Environ Pathol Toxicol Oncol. 2019;38:21–8.
Kim SM, An JY, Byeon SJ, et al. Prognostic value of mismatch repair deficiency in patients with advanced gastric cancer, treated by surgery and adjuvant 5-fluorouracil and leucovorin chemoradiotherapy. Eur J Surg Oncol. 2020;46:189–94.
Corso G, Pedrazzani C, Marrelli D, Pascale V, Pinto E, Roviello F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg. 2009;144:722–7.
Kim JY, Shin NR, Kim A, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol. 2013;47:28–35.
Polom K, Marano L, Marrelli D, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159–67.
Hashimoto T, Kurokawa Y, Takahashi T, et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer. 2019;22:785–92.
do Nascimento CN, Mascarenhas-Lemos L, Silva JR, et al. EBV and MSI status in gastric cancer: does it matter? Cancers (Basel). 2022;15(1):74. https://doi.org/10.3390/cancers15010074.
Kohlruss M, Grosser B, Krenauer M, et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: role of Epstein-Barr virus infection and high- and low-microsatellite instability. J Pathol Clin Res. 2019;5:227–39.
Kohlruss M, Ott K, Grosser B, et al. Sexual difference matters: females with high microsatellite instability show increased survival after neoadjuvant chemotherapy in gastric cancer. Cancers (Basel). 2021;13(5):1048. https://doi.org/10.3390/cancers13051048.
Cai Z, Rui W, Li S, et al. Microsatellite status affects tumor response and survival in patients undergoing neoadjuvant chemotherapy for clinical stage III gastric cancer. Front Oncol. 2020;10:614785.
Vos EL, Maron SB, Krell RW, et al. Survival of locally advanced MSI-high gastric cancer patients treated with perioperative chemotherapy: a retrospective cohort study. Ann Surg. 2023;277:798–805.
Zhu L, Li Z, Wang Y, Zhang C, Liu Y, Qu X. Microsatellite instability and survival in gastric cancer: a systematic review and meta-analysis. Mol Clin Oncol. 2015;3:699–705.
Falchetti M, Saieva C, Lupi R, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–32.
Lee HS, Choi SI, Lee HK, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–40.
Quaas A, Biesma HD, Wagner AD, et al. Microsatellite instability and sex differences in resectable gastric cancer—A pooled analysis of three European cohorts. Eur J Cancer. 2022;173:95–104.
Martinez-Ciarpaglini C, Fleitas-Kanonnikoff T, Gambardella V, et al. Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications. ESMO Open. 2019;4: e000470.
Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–8.
El Helali A, Tao J, Wong CHL, et al. A meta-analysis with systematic review: efficacy and safety of immune checkpoint inhibitors in patients with advanced gastric cancer. Front Oncol. 2022;12:908026.
Pietrantonio F, Randon G, Di Bartolomeo M, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6:100036.
Lorenzen S, Götze TO, Thuss-Patience P, et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 trial. J Clin Oncol. 2024;42:410–20.
Wu H, Ma W, Jiang C, et al. Heterogeneity and adjuvant therapeutic approaches in MSI-H/dMMR resectable gastric cancer: emerging trends in immunotherapy. Ann Surg Oncol. 2023;30:8572–87.
Rha SY, Oh DY, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–95.
Shitara K, Rha SY, Wyrwicz LS, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25:212–24.
Janjigian YY, Al-Batran S-E, Wainberg ZA, et al. Pathological complete response to 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) with or without durvalumab in resectable gastric and gastroesophageal junction cancer: subgroup analysis by region from the phase 3 randomized, double-blind MATTERHORN study. J Clin Oncol. 2024;42(suppl 3):LBA246. https://doi.org/10.1200/JCO.2024.42.3_suppl.LBA246.
Capelle LG, Van Grieken NC, Lingsma HF, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487–92.
The National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Gastric Cancer. 2024. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.version 1. Accessed 03 Apr 2024.
Polom K, Marrelli D, Roviello G, et al. Molecular key to understand the gastric cancer biology in elderly patients-The role of microsatellite instability. J Surg Oncol. 2017;115:344–50.
Arai T, Sakurai U, Sawabe M, et al. Frequent microsatellite instability in papillary and solid-type, poorly differentiated adenocarcinomas of the stomach. Gastric Cancer. 2013;16:505–12.
Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6.
Arai T, Matsuda Y, Aida J, Takubo K, Ishiwata T. Solid-type poorly differentiated adenocarcinoma of the stomach: clinicopathological and molecular characteristics and histogenesis. Gastric Cancer. 2019;22:314–22.
Silva JR, Mascarenhas-Lemos L, do Neto Nascimento C, et al. Role of endoscopic biopsies and morphologic features in predicting microsatellite instability status in gastric cancer: a multicenter comparative study of endoscopic biopsies and surgical specimens. Am J Surg Pathol. 2023;47:990–1000.
Derks S, de Klerk LK, Xu X, et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol. 2020;31:1011–20.
Giampieri R, Maccaroni E, Mandolesi A, et al. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer. 2017;20:156–63.
Derks S, Liao X, Chiaravalli AM, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925–32.
Angell HK, Lee J, Kim KM, et al. PD-L1 and immune infiltrates are differentially expressed in distinct subgroups of gastric cancer. Oncoimmunology. 2019;8: e1544442.
Cho J, Chang YH, Heo YJ, et al. Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open. 2018;3: e000326.
Chiaravalli AM, Feltri M, Bertolini V, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006;448:344–53.
Wang Z, Wang X, Xu Y, et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022;20:133.
Kim KJ, Lee KS, Cho HJ, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014;45:285–93.
Koh J, Ock CY, Kim JW, et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget. 2017;8:26356–67.
Ock CY, Keam B, Kim S, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res. 2016;22:2261–70.
Koh J, Nam SK, Roh H, et al. Somatic mutational profiles of stage II and III gastric cancer according to tumor microenvironment immune type. Genes Chromosomes Cancer. 2019;58:12–22.
Gullo I, Oliveira P, Athelogou M, et al. New insights into the inflamed tumor immune microenvironment of gastric cancer with lymphoid stroma: from morphology and digital analysis to gene expression. Gastric Cancer. 2019;22:77–90.
Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–15.
Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS ONE. 2017;12: e0182692.
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9.
Ooki A, Akagi K, Yatsuoka T, et al. Combined microsatellite instability and BRAF gene status as biomarkers for adjuvant chemotherapy in stage III colorectal cancer. J Surg Oncol. 2014;110:982–8.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18.
Nakajima T, Akiyama Y, Shiraishi J, et al. Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int J Cancer. 2001;94:208–11.
Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8:49–58.
Yamamoto H, Watanabe Y, Maehata T, et al. An updated review of gastric cancer in the next-generation sequencing era: insights from bench to bedside and vice versa. World J Gastroenterol. 2014;20:3927–37.
Kim TM, Jung SH, Kim MS, et al. The mutational burdens and evolutionary ages of early gastric cancers are comparable to those of advanced gastric cancers. J Pathol. 2014;234:365–74.
Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82.
Lim CH, Cho YK, Kim SW, et al. The chronological sequence of somatic mutations in early gastric carcinogenesis inferred from multiregion sequencing of gastric adenomas. Oncotarget. 2016;7:39758–67.
Gutierrez-Gonzalez L, Graham TA, Rodriguez-Justo M, et al. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology. 2011;140:1251–60.
Lipinski KA, Barber LJ, Davies MN, Ashenden M, Sottoriva A, Gerlinger M. Cancer evolution and the limits of predictability in precision cancer medicine. Trends Cancer. 2016;2:49–63.
Liu J, McCleland M, Stawiski EW, et al. Integrated exome and transcriptome sequencing reveals ZAK isoform usage in gastric cancer. Nat Commun. 2014;5:3830.
Zhu C, Zhu L, Gu Y, et al. Genomic profiling reveals the molecular landscape of gastrointestinal tract cancers in chinese patients. Front Genet. 2021;12:608742.
Yuza K, Nagahashi M, Ichikawa H, et al. Activin a receptor type 2A mutation affects the tumor biology of microsatellite instability-high gastric cancer. J Gastrointest Surg. 2021;25:2231–41.
Zang ZJ, Cutcutache I, Poon SL, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–4.
Ichikawa H, Nagahashi M, Shimada Y, et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017;9:93.
Kim Y, Cho MY, Kim J, Kim SN, Oh SC, Lee KA. Profiling cancer-associated genetic alterations and molecular classification of cancer in Korean gastric cancer patients. Oncotarget. 2017;8:69888–905.
Wang K, Kan J, Yuen ST, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–23.
Polom K, Das K, Marrelli D, et al. KRAS mutation in gastric cancer and prognostication associated with microsatellite instability status. Pathol Oncol Res. 2019;25:333–40.
Corso G, Velho S, Paredes J, et al. Oncogenic mutations in gastric cancer with microsatellite instability. Eur J Cancer. 2011;47:443–51.
Oliveira C, Seruca R, Seixas M, Sobrinho-Simões M. The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes”: a study of the TGFbeta RII, IGFII R, and BAX genes. Am J Pathol. 1998;153:1211–9.
Iacopetta BJ, Soong R, House AK, Hamelin R. Gastric carcinomas with microsatellite instability: clinical features and mutations to the TGF-beta type II receptor, IGFII receptor, and BAX genes. J Pathol. 1999;187:428–32.
Fang WL, Chen MH, Huang KH, et al. The clinicopathological features and genetic mutations in gastric cancer patients according to EMAST and MSI status. Cancers (Basel). 2020;12:551. https://doi.org/10.3390/cancers12030551.
Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787–91.
Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62:2447–54.
Mori Y, Sato F, Selaru FM, et al. Instabilotyping reveals unique mutational spectra in microsatellite-unstable gastric cancers. Cancer Res. 2002;62:3641–5.
Nagarajan N, Bertrand D, Hillmer AM, et al. Whole-genome reconstruction and mutational signatures in gastric cancer. Genome Biol. 2012;13:R115.
Piao Z, Fang W, Malkhosyan S, et al. Frequent frameshift mutations of RIZ in sporadic gastrointestinal and endometrial carcinomas with microsatellite instability. Cancer Res. 2000;60:4701–4.
Pan KF, Lu YY, Liu WG, Zhang L, You WC. Detection of frameshift mutations of RIZ in gastric cancers with microsatellite instability. World J Gastroenterol. 2004;10:2719–22.
Menoyo A, Alazzouzi H, Espín E, Armengol M, Yamamoto H, Schwartz S Jr. Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727–30.
Tan KT, Yeh CN, Chang YC, et al. PRKDC: new biomarker and drug target for checkpoint blockade immunotherapy. J Immunother Cancer. 2020;8: e000485. https://doi.org/10.1136/jitc-2019-000485.
Albacker LA, Wu J, Smith P, et al. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS ONE. 2017;12: e0176181.
Yamamoto H, Perez-Piteira J, Yoshida T, et al. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348–57.
Liu F, Zhong F, Wu H, et al. Prevalence and associations of Beta2-microglobulin mutations in MSI-H/dMMR cancers. Oncologist. 2023;28:e136–44.
Zhong D, He Q, Wang X, Wang C, Ma T. 140P KMT2C/KMT2D (KMT2C/D): promising biomarkers for immunotherapy in gastric cancer. Ann Oncol. 2020;31:S295–6.
Maruvka YE, Mouw KW, Karlic R, et al. Analysis of somatic microsatellite indels identifies driver events in human tumors. Nat Biotechnol. 2017;35:951–9.
Pu X, Fu Y, Sun Q, et al. NTRK gene alterations were enriched in hepatoid or enteroblastic differentiation type of gastric cancer. J Clin Pathol. 2023. https://doi.org/10.1136/jcp-2023-208865.
Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51.
Mandal R, Samstein RM, Lee KW, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. 2019;364:485–91.
Zhao R, Wan Q, Wang Y, et al. M1-like TAMs are required for the efficacy of PD-L1/PD-1 blockades in gastric cancer. Oncoimmunology. 2020;10:1862520.
Li Y, Xu C, Wang B, et al. Proteomic characterization of gastric cancer response to chemotherapy and targeted therapy reveals new therapeutic strategies. Nat Commun. 2022;13:5723.
Li B, Jiang Y, Li G, Fisher GA Jr, Li R. Natural killer cell and stroma abundance are independently prognostic and predict gastric cancer chemotherapy benefit. JCI Insight. 2020;5:e136570. https://doi.org/10.1172/jci.insight.136570.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.
Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45.
Hawn MT, Umar A, Carethers JM, et al. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 1995;55:3721–5.
Fink D, Nebel S, Norris PS, et al. The effect of different chemotherapeutic agents on the enrichment of DNA mismatch repair-deficient tumour cells. Br J Cancer. 1998;77:703–8.
Li LS, Morales JC, Veigl M, et al. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br J Pharmacol. 2009;158:679–92.
Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–201.
Pereira MA, Dias AR, Ramos M, et al. Gastric cancer with microsatellite instability displays increased thymidylate synthase expression. J Surg Oncol. 2022;126:116–24.
Valentini AM, Armentano R, Pirrelli M, Caruso ML. Chemotherapeutic agents for colorectal cancer with a defective mismatch repair system: the state of the art. Cancer Treat Rev. 2006;32:607–18.
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57.
Morton D, Seymour M, Magill L, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol. 2023;41:1541–52.
Haag GM, Czink E, Ahadova A, et al. Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer. 2019;144:1697–703.
Vos EL, Maron SB, Krell RW, et al. The interaction between microsatellite instability high (MSI-high) gastric cancer and chemotherapy on survival. J Clin Oncol. 2021;39:244–244.
Yang Y, Shi Z, Bai R, Hu W. Heterogeneity of MSI-H gastric cancer identifies a subtype with worse survival. J Med Genet. 2021;58:12–9.
Cohen R, Taieb J, Fiskum J, et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol. 2021;39:642–51.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304.
Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–20.
Lordick F. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020;21:203.
Smyth EC. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020;21:204.
Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47.
Ooki A, Yamaguchi K. The dawn of precision medicine in diffuse-type gastric cancer. Ther Adv Med Oncol. 2022;14:17588359221083048.
Association JGC. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1–25.
Kim TH, Kim IH, Kang SJ, et al. Korean practice guidelines for gastric cancer 2022: an evidence-based multidisciplinary approach. J Gastric Cancer. 2023;23:3–106.
Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80.
Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33.
Fei S, Lu Y, Chen J, et al. Efficacy of PD-1 inhibitors in first-line treatment for advanced gastroesophageal junction and gastric cancer by subgroups: a systematic review and meta-analysis. Chemotherapy. 2023;68:197-209.
Kundel Y, Sternschuss M, Moore A, Perl G, Brenner B, Goldvaser H. Efficacy of immune-checkpoint inhibitors in metastatic gastric or gastroesophageal junction adenocarcinoma by patient subgroups: a systematic review and meta-analysis. Cancer Med. 2020;9:7613–25.
Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. 2022;8:1456–65.
Chen C, Zhang F, Zhou N, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. 2019;8: e1581547.
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40.
Xu J, Jiang H, Pan Y, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. 2023;330:2064–74.
Moehler MH, Kato K, Arkenau H-T, et al. Rationale 305: phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 2023;41:286–286.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75.
McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9.
Hwang HS, Kim D, Choi J. Distinct mutational profile and immune microenvironment in microsatellite-unstable and POLE-mutated tumors. J Immunother Cancer. 2021;9(10):e002797. https://doi.org/10.1136/jitc-2021-002797.
Iwasaki A, Shinozaki-Ushiku A, Kunita A, et al. Human leukocyte antigen class I deficiency in gastric carcinoma: an adaptive immune evasion strategy most common in microsatellite instable tumors. Am J Surg Pathol. 2021;45:1213–20.
Chida K, Kawazoe A, Suzuki T, et al. Transcriptomic profiling of MSI-H/dMMR gastrointestinal tumors to identify determinants of responsiveness to anti-PD-1 therapy. Clin Cancer Res. 2022;28:2110–7.
Chida K, Kawazoe A, Kawazu M, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res. 2021;27:3714–24.
Huang YK, Wang M, Sun Y, et al. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat Commun. 2019;10:3928.
Chen Y, Jia K, Sun Y, et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13:4851.
Park Y, Seo AN, Koh J, et al. Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. Oncoimmunology. 2021;10:1954761.
Anderson AC, Joller N, Kuchroo VK. Lag-3, tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004.
Muro K, Kawakami H, Kadowaki S, et al. 1513MO A phase II study of nivolumab plus low dose ipilimumab as first -line therapy in patients with advanced gastric or esophago-gastric junction MSI-H tumor: First results of the NO LIMIT study (WJOG13320G/CA209-7W7). Ann Oncol. 2023;34:S852–3.
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51.
Schmidt EV, Chisamore MJ, Chaney MF, et al. Assessment of clinical activity of PD-1 checkpoint inhibitor combination therapies reported in clinical trials. JAMA Netw Open. 2020;3: e1920833.
Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138.
Gebert J, Gelincik O, Oezcan-Wahlbrink M, et al. Recurrent frameshift neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a lynch syndrome mouse model. Gastroenterology. 2021;161:1288-1302.e1213.
Kloor M, Reuschenbach M, Pauligk C, et al. A frameshift peptide neoantigen-based vaccine for mismatch repair-deficient cancers: a phase I/IIa clinical trial. Clin Cancer Res. 2020;26:4503–10.
Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86.
Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25:454–61.
Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm. Phase II Study J Clin Oncol. 2018;36:3353–60.
Verschoor YL, Berg JVD, Beets G, et al. Neoadjuvant nivolumab, ipilimumab, and celecoxib in MMR-proficient and MMR-deficient colon cancers: final clinical analysis of the NICHE study. J Clin Oncol. 2022;40:3511–3511.
Chalabi M, Verschoor YL, van den Berg J, et al. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study. Ann Oncol. 2022;33:S1389.
Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–76.
Terashima M, Kang Y-K, Kim Y-W, et al. ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as postoperative adjuvant treatment for pathological stage III (pStage III) gastric or gastroesophageal junction (G/GEJ) cancer. J Clin Oncol. 2023;41:4000–4000.
Li S, Xu Q, Dai X, et al. Neoadjuvant therapy with immune checkpoint inhibitors in gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2023;30:3594–602.
Verschoor YL, van de Haar J, van den Berg JG, et al. Neoadjuvant atezolizumab plus chemotherapy in gastric and gastroesophageal junction adenocarcinoma: the phase 2 PANDA trial. Nat Med. 2024;30:519–30.
Li S, Yu W, Xie F, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8.
Lin JX, Tang YH, Zheng HL, et al. Neoadjuvant camrelizumab and apatinib combined with chemotherapy versus chemotherapy alone for locally advanced gastric cancer: a multicenter randomized phase 2 trial. Nat Commun. 2024;15:41.
André T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2023;41:255–65.
Pietrantonio F, Raimondi A, Lonardi S, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41:358–358.
Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–99.
Friedman J, Moore EC, Zolkind P, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2020;26:679–89.
Chung J, Maruvka YE, Sudhaman S, et al. DNA polymerase and mismatch repair exert distinct microsatellite instability signatures in normal and malignant human cells. Cancer Discov. 2021;11:1176–91.
Rampias T, Karagiannis D, Avgeris M, et al. The lysine-specific methyltransferase KMT2C/MLL3 regulates DNA repair components in cancer. EMBO Rep. 2019;20: e46821.
Mittal P, Roberts CWM. The SWI/SNF complex in cancer—biology, biomarkers and therapy. Nat Rev Clin Oncol. 2020;17:435–48.
Williamson CT, Miller R, Pemberton HN, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837.
Kim KH, Kim W, Howard TP, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21:1491–6.
Shen J, Peng Y, Wei L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–67.
Helming KC, Wang X, Wilson BG, et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat Med. 2014;20:251–4.
Ogiwara H, Takahashi K, Sasaki M, et al. Targeting the vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell. 2019;35:177-190.e178.
Bitler BG, Wu S, Park PH, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19:962–73.
Lo YH, Kolahi KS, Du Y, et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11:1562–81.
Nargund AM, Xu C, Mandoli A, et al. Chromatin rewiring by mismatch repair protein MSH2 alters cell adhesion pathways and sensitivity to BET inhibition in gastric cancer. Cancer Res. 2022;82:2538–51.
Bai J, Yang B, Shi R, et al. Could microtubule inhibitors be the best choice of therapy in gastric cancer with high immune activity: mutant DYNC1H1 as a biomarker. Aging (Albany NY). 2020;12:25101–19.
Chan EM, Shibue T, McFarland JM, et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature. 2019;568:551–6.
Cortes-Cros M, Moebitz H, Barbe S, et al. Abstract PR007: discovery of HRO761, a novel, first-in-class clinical stage WRN inhibitor with potent and selective anti-tumor activity in cancers with microsatellite instability. Mol Cancer Ther. 2023;22:PR007–PR007.
Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–43.
van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer. 2013;108:1495–501.
Moore AR, Rosenberg SC, McCormick F, Malek S. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19:533–52.
Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–51.
Lee D, Yu EJ, Ham IH, Hur H, Kim YS. AKT inhibition is an effective treatment strategy in ARID1A-deficient gastric cancer cells. Onco Targets Ther. 2017;10:4153–9.
Fan JP, Qian J, Zhao YJ. The loss of PTEN expression and microsatellite stability (MSS) were predictors of unfavorable prognosis in gastric cancer (GC). Neoplasma. 2020;67:1359–66.
Chung YJ, Kim KM, Choi JR, Choi SW, Rhyu MG. Relationship between intratumor histological heterogeneity and genetic abnormalities in gastric carcinoma with microsatellite instability. Int J Cancer. 1999;82:782–8.
Sardet C, Vidal M, Cobrinik D, et al. E2F–4 and E2F–5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci U S A. 1995;92:2403–7.
Yuan Z, Shin J, Wilson A, et al. An A13 repeat within the 3’-untranslated region of epidermal growth factor receptor (EGFR) is frequently mutated in microsatellite instability colon cancers and is associated with increased EGFR expression. Cancer Res. 2009;69:7811–8.
Souza RF, Appel R, Yin J, et al. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996;14:255–7.
Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592–600.
Lei Y, Chen L, Zhang G, et al. MicroRNAs target the Wnt/β-catenin signaling pathway to regulate epithelial-mesenchymal transition in cancer (Review). Oncol Rep. 2020;44:1299–313.
Giannakis M, Hodis E, Jasmine MuX, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46:1264–6.
Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–9.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi pp. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–8.
Li Y, Yang X, Zhu W, et al. SWI/SNF complex gene variations are associated with a higher tumor mutational burden and a better response to immune checkpoint inhibitor treatment: a pan-cancer analysis of next-generation sequencing data corresponding to 4591 cases. Cancer Cell Int. 2022;22:347.
Kadoch C, Hargreaves DC, Hodges C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45:592–601.
Shen J, Ju Z, Zhao W, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–62.
Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49:825–37.
Dorighi KM, Swigut T, Henriques T, et al. Mll3 and Mll4 facilitate enhancer RNA synthesis and transcription from promoters independently of H3K4 monomethylation. Mol Cell. 2017;66:568-576.e564.
Fagan RJ, Dingwall AK. COMPASS ascending: emerging clues regarding the roles of MLL3/KMT2C and MLL2/KMT2D proteins in cancer. Cancer Lett. 2019;458:56–65.
Mzoughi S, Tan YX, Low D, Guccione E. The role of PRDMs in cancer: one family, two sides. Curr Opin Genet Dev. 2016;36:83–91.
Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15:166–80.
Goldstein M, Kastan MB. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–43.
Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, et al. ATM activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–45.
D’Errico M, de Rinaldis E, Blasi MF, et al. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009;45:461–9.
Goodwin JF, Knudsen KE. Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 2014;4:1126–39.
Cerniglia M, Xiu J, Grothey A, et al. Association of DNA damage response and repair genes (DDR) mutations and microsatellite instability (MSI), PD-L1 expression, tumor mutational burden (TMB) in gastroesophageal cancers. J Clin Oncol. 2019;37:60–60.
Kim HS, Choi SI, Min HL, Kim MA, Kim WH. Mutation at intronic repeats of the ataxia-telangiectasia mutated (ATM) gene and ATM protein loss in primary gastric cancer with microsatellite instability. PLoS ONE. 2013;8: e82769.
Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201.
Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39.
Halle S, Halle O, Förster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017;38:432–43.
Echterdiek F, Janikovits J, Staffa L, et al. Low density of FOXP3-positive T cells in normal colonic mucosa is related to the presence of beta2-microglobulin mutations in Lynch syndrome-associated colorectal cancer. Oncoimmunology. 2016;5: e1075692.
Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–29.
Bortolomeazzi M, Keddar MR, Montorsi L, et al. Immunogenomics of colorectal cancer response to checkpoint blockade: analysis of the KEYNOTE 177 trial and validation cohorts. Gastroenterology. 2021;161:1179–93.
Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479–86.
Fuchs CS, Özgüroğlu M, Bang Y-J, et al. The association of molecular biomarkers with efficacy of pembrolizumab versus paclitaxel in patients with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol. 2020;38:4512–4512.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–37.
Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153:1107-1119.e1110.
Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51.
Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96.
Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27.
Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34.
Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75.
Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–80.
Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82.
Miyamoto N, Yamamoto H, Taniguchi H, et al. Differential expression of angiogenesis-related genes in human gastric cancers with and those without high-frequency microsatellite instability. Cancer Lett. 2007;254:42–53.
Mo DC, Luo PH, Huang SX, Wang HL, Huang JF. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: a systematic review. Int Immunopharmacol. 2021;91:107281.
Peri SS, Narayanaa YK, Hubert TD, et al. Navigating tumour microenvironment and Wnt signalling crosstalk: implications for advanced cancer therapeutics. Cancers (Basel). 2023;15:5847. https://doi.org/10.3390/cancers15245847.
Jiang X, Hao HX, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:12649–54.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5.
Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8.
Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94.
Endo E, Okayama H, Saito K, et al. A TGFβ-dependent stromal subset underlies immune checkpoint inhibitor efficacy in DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Mol Cancer Res. 2020;18:1402–13.
Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882-897.e811.
Bitler BG, Aird KM, Garipov A, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–8.
Yamada L, Saito M, Thar Min AK, et al. Selective sensitivity of EZH2 inhibitors based on synthetic lethality in ARID1A-deficient gastric cancer. Gastric Cancer. 2021;24:60–71.
Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23:78–94.
Mouw KW, Goldberg MS, Konstantinopoulos PA, D’Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017;7:675–93.
McGrail DJ, Garnett J, Yin J, et al. Proteome instability is a therapeutic vulnerability in mismatch repair-deficient cancer. Cancer Cell. 2020;37:371-386.e312.
Funding
This study received no grant support.
Author information
Authors and Affiliations
Contributions
A.O. developed the conceptual frameworks, searched the literature, and wrote the manuscript. H.O., K. Yoshino, and K. Yamaguchi revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
AO received speaker honoraria from Bristol-Myers Squibb, Ono Pharmaceutical, Daiichi Sankyo, and Taiho Pharmaceutical Co. K. Yamaguchi received speaker honoraria from Chugai Pharmaceutical Co. Ltd., Bristol-Myers Squibb, Merck Serono, Taiho Pharmaceutical Co., Takeda, and Eli Lilly; a consultant fee from Takeda Pharmaceutical Co. Ltd.; honoraria from Tsumura Co. Ltd., Nihon Kayaku Co. Ltd., and Chugai Pharmaceutical Co. Ltd; research grants from Sumitomo Dainippon Pharma, Gilead Sciences, MSD, Boehringer Ingelheim, Daiichi Sankyo, and Chugai Pharmaceutical Co. Ltd; and speaker honoraria, research grants, and scholarship grants from Ono Pharmaceutical, Yakult Honsha Co., Ltd., and Sanofi.
Consent for publication
N/A.
Ethical approval
N/A
Informed consent
N/A
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ooki, A., Osumi, H., Yoshino, K. et al. Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair. Gastric Cancer (2024). https://doi.org/10.1007/s10120-024-01523-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10120-024-01523-4