Abstract
Introduction
Contour maps enable risk classification of GIST recurrence in individual patients within 10 postoperative years. Although contour maps have been referred to in Japanese guidelines, their usefulness and role in determining indications for adjuvant therapy is still unclear in Japanese patients. The aims of this study are to investigate the validity of contour maps in Japanese patients with GIST and explore the new strategy for adjuvant therapy.
Materials and methods
A total of 1426 Japanese GIST patients who were registered to the registry by the Kinki GIST Study Group between 2003 and 2012 were analyzed. Patients who had R0 surgery without perioperative therapy were included in this study. The accuracy of contour maps was validated.
Results
Overall, 994 patients have concluded this study. Using contour maps, we validated the patients. The 5-year recurrence-free survival rates of patients within the GIST classification groups of 0–10%, 10–20%, 20–40%, 40–60%, 60–80%, 80–90%, and 90–100% were 98.1%, 96.6%, 92.3%, 48.0%, 37.3%, 41.0% and 42.4%, respectively. We confirmed that this classification by contour maps was well reflected recurrence prediction. Further, in the high-risk group stratified by the modified National Institutes of Health consensus criteria (m-NIHC), the 10-year RFS rate was remarkably changed at a cutoff of 40% (0–40% group vs. 40–100% group: 88.7% vs. 50.3%, p < 0.001).
Conclusion
Contour maps are effective in predicting individual recurrence rates. And it may be useful for the decision of individual strategy for high-risk patients combined with m-NIHC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal stromal tumors (GISTs) represent the most common type of gastrointestinal mesenchymal tumors and are detected most often in the stomach, followed by the small intestine and other sites in the colon, esophagus, and peritoneal cavity [1,2,3,4,5]. Surgical resection with a negative margin remains the only therapeutic modality for cure.
Adjuvant therapy has recently been established for patients with a high-risk of recurrence GISTs [6, 7]. Three randomized phase III trials evaluating the efficacy of adjuvant therapy with imatinib mesylate (IM) demonstrated the efficacy of adjuvant therapy with IM for high-risk GIST recurrence after resection and revealed that recurrence-free survival (RFS) was significantly prolonged in patients treated with IM for a duration of 1–3 years, as compared with that in controls [7,8,9,10]. Nonetheless, the eligibility criteria for randomization of patients in each trial were different. The first trial (i.e., the American College of Surgeons Oncology Group Z9001 study) targeted patients with GIST measuring ≥ 3 cm in size and reported that adjuvant IM treatment for 1 year improved the RFS rate, as compared with that in controls. Following this study, patients in the high-risk group according to the National Institutes of Health consensus criteria (NIHC) and patients with tumor rupture were randomized in the Scandinavian Sarcoma Group XVIII/Arbeitsgemeinschaft Internistische Onkologie study [9]. The European Organisation for Research and Treatment of Cancer 62,024 study was the largest phase III trial that targeted patients with primary GIST who had high and intermediate risk based on the NIHC [8]. For these reasons, there is no consensus on the indications for adjuvant therapy. Therefore, predicting the patient-specific risk of GIST recurrence plays a crucial role in determining indications for adjuvant therapy.
Several risk-stratification systems for analyzing patients after radical resection of GISTs have been proposed. The NIHC was proposed in 2002 and have been widely used globally since then [11]. This classification is based on tumor size and the number of mitotic counts per 50 high-power fields (HPFs). However, further study showed that the location of GISTs was also one of the important and independent prognostic factors for recurrence in patients after radical resection. Miettinen et al. proposed the Armed Forces Institute of Pathology Criteria; this classification involved the location of GISTs in addition to the tumor size and number of mitotic counts [3]. The presence of tumor rupture, which is a strong adverse prognostic factor for GISTs and potentially important in determining patient prognosis [12,13,14], is absent in these classification systems. Therefore, Joensuu et al. proposed the modified NIHC (m-NIHC) [15], which considers ruptured GISTs as high-risk tumors irrespective of other features since tumor rupture is a clinically malignant factor for recurrence. Previously, we compared these three risk classifications and reported that m-NIHC exhibited the highest sensitivity for predicting recurrence; hence, we proposed it for the identification of candidates for adjuvant therapy [14]. Despite tumor size and mitotic count showing a nonlinear association with the risk of recurrence, these were estimated by linear modelling. Therefore, current methods of estimating individuals’ survival outcomes were faulty.
Joensuu et al. proposed contour maps, which enable risk classification of recurrence in individual patients. Contour maps consider tumor size, location, mitotic count, and rupture and consider the tumor size and mitotic count as continuous nonlinear variables [16]. They reported that contour maps were more detailed than other criteria with respect to the 10-year risk of GIST recurrence and were appropriate for estimating the individualized outcomes. However, although contour maps have been referred to in Japanese guidelines [17], their validity has not been confirmed in Asian patients. Therefore, the present study aimed to clarify the validity of contour maps in Japanese patients with GIST and explore the new strategy for adjuvant therapy.
Methods
Study design and patients
The GIST registry protocol was designed by the Kinki GIST Study Group. In this study, 1426 patients with GISTs diagnosed by a pathologist at each institution were retrospectively and prospectively enrolled from 39 hospitals between 2003 and 2012. In total, 737 patients were retrospectively enrolled between January 2003 and December 2007, and 689 patients were prospectively enrolled from January 2008. The eligible patients were required to have tumor morphology compatible with GIST and positive immunostaining for the KIT protein. Only tumors that were completely removed macroscopically during surgery were considered eligible. Patients with distant metastases at the time of primary therapy were excluded. In addition, since the pathological features of patients after neoadjuvant therapy might be altered, patients who received neoadjuvant therapy were also excluded from the study. Finally, since the adjuvant therapy has been reported to influence postoperative results, patients who received adjuvant therapy were also excluded from the study.
The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved this study (No. 18424-2), and the institutional review board at each other participating institution approved the study protocol.
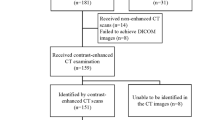
Most postoperative follow-ups were performed using contrast-enhanced computed tomography (CT) to detect recurrence and metastasis, per the Japanese guidelines for GISTs [17]. Data on patients’ prognoses, characteristics, and clinicopathological features, including age, sex, tumor location, size, mitotic count, and tumor rupture, were reviewed from medical reports. Maximum tumor size was measured using operative specimens. Data on histopathological features and the number of mitoses per 50 HPFs were obtained by examining specimens stained with hematoxylin and eosin. Mitoses were counted at the highest power, and the mean value was used for analysis after counting the fields twice.
Evaluation methods for estimating GIST recurrence
The risk of recurrence in patients with GISTs was estimated using m-NIHC and contour maps [15, 16]. The m-NIHC divides the risk of recurrence into the following four categories: very low, low, intermediate, and high risk. Contour maps predict the GIST recurrence rate at 10 years after surgery and categorize GISTs into seven groups (0–10%, 10–20%, 20–40%, 40–60%, 60–80%, 80–90%, and 90–100%) according to tumor size, mitotic count (per 50 HPFs), location, and rupture [16]. Tumor size > 25 cm and mitotic count > 50 per 50 HPFs were assumed to be equivalent to 25 cm and 50 HPF, respectively.
Statistical analysis
RFS was calculated from the date of surgery until the date of the first recurrence. Overall survival (OS) was calculated from the date of surgery until the date of death. The censored was the last date of survival confirmation. These were calculated for various subsets of prognostic factors using the Kaplan–Meier method and log-rank test. The χ2 test was used to estimate sensitivity. Two-sided p values of < 0.05 were considered as statistically significant. Statistical analyses were performed using JMP® Pro 16.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
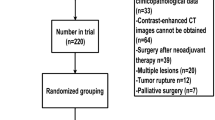
The study enrolled 1426 GIST patients from 39 hospitals. First, 173 patients with insufficient data were excluded. Furthermore, 26 patients who received non-surgical treatment and 29 patients who received neoadjuvant therapy were excluded. Next, 35 patients who underwent non-curative resection (R2) based on operative findings were excluded. There were 1163 patients who underwent curative resection (R0 and R1). Of these patients, 45 with synchronous metastases and 124 patients who received adjuvant therapy were excluded. Ultimately, 994 patients were included in this study (Fig. 1). The clinicopathological features of 994 patients are shown (Table 1). The median observation period was 6.1 years (range 0.02–20.3 years). Tumor locations included 779 GISTs in the stomach, 168 in the duodenum and small intestine, 29 in the large intestine, and 11 in the esophagus. The median tumor size was 3.3 cm (range 0.1–70 cm). The median number of mitoses per 50 HPFs was 2 (range 0–160). Tumor rupture was observed in 18 patients (1.8%). In total, 76 (7.6%) cases of recurrence were identified. Based on the m-NIHC, 183 patients (18.4%) were classified as having a very low risk, 456 (45.9%) as having a low risk, 144 (14.5%) as having an intermediate risk, and 211 (21.2%) as having a high risk. The recurrence rate for each group was as follows: 1.1% (2 patients) in the very low-risk group, 2.0% (9 patients) in the low-risk group, 4.9% (7 patients) in the intermediate-risk group, and 27.5% (58 patients) in the high-risk group. Based on the contour maps, 404 patients (40.6%) were classified as the 0–10% group, 297 patients (29.9%) as the 10–20% group, 164 patients (16.5%) as the 20–40% group, 51 patients (5.1%) as the 40–60% group, 23 patients (2.3%) as the 60–80% group, 17 patients (1.7%) as the 80–90% group, and 38 patients (3.8%) as the 90–100% group.
Risk-group stratification and outcome analysis for the m-NIHC and contour maps in patients
We validated these 994 patients using the m-NIHC and contour maps. Recurrence occurred in 76 patients (7.6%). Based on the m-NIHC, 183 patients were classified as having a very low risk, 456 as having a low risk, 144 as having an intermediate risk, and 211 as having a high risk. The OS rate of the very low-risk group was similar to that of the high-risk group (Fig. 2A). The high-risk group showed significantly poorer prognosis (10-year RFS rate: 62.9%) than the other three risk groups (Fig. 2B). Next, we evaluated the risk of GIST recurrence using contour maps. Figure 3 shows only recurrent cases plotted into contour maps according to each tumor characteristic. There were 30 cases of recurrence in stomach GISTs without rupture, 4 in stomach GISTs with rupture, 35 in non-stomach GISTs without rupture, 6 in non-stomach GISTs with rupture, and 1 in extra-gastrointestinal GISTs without rupture (Fig. 3A–E). Evaluation of prognosis according to each risk classification using contour maps is shown in Fig. 4. The OS and RFS rates are shown in Fig. 4A, B. The 5-year RFS rates of patients within each of the GIST classification groups of 0–10%, 10–20%, 20–40%, 40–60%, 60–80%, 80–90%, and 90–100% were 98.1%, 96.6%, 92.3%, 48.0%, 37.3%, 41.0% and 42.4% respectively. We confirmed that the higher prediction group showed higher recurrence.
Recurrent cases plotted on contour maps. A Stomach gastrointestinal stromal tumors (GISTs) with no rupture, B non-stomach GISTs with no rupture, C extra-gastrointestinal stromal tumor with no rupture, D stomach GISTs with rupture, and E non-stomach GISTs with rupture. Tumor size over 25 cm and mitotic count over 50 per 50 high-power fields (HPFs) are assumed to be equivalent to 25 cm and 50 HPFs, respectively. This figure was referred from Joensuu et al. [16]
Detailed analysis for high-risk classification based on the m-NIHC using the contour maps
Patients in the high-risk group based on the m-NIHC were reclassified using the contour maps (Fig. 5). In this population, a cutoff value of 40% also showed a significant difference in RFS, with the 10-year RFS rate being 88.7% in the 0–40% group and 50.3% in the 40–100% group (p < 0.001).
Discussion
The Kinki GIST registry comprises the largest Japanese population in retrospective and prospective studies [14, 18,19,20,21,22]. Because this cohort study included consecutive patients in each hospital, this patient population reflects the characteristics of real-world Japanese patients with GIST. The proportion of patients with gastric GISTs was higher than that reported by previous international studies [12, 16]. In addition, there were fewer high-risk GISTs and more low-risk GISTs based on the m-NIHC, as compared with those in international reports [15, 16, 23]. This might be attributable to the well-established screening system for gastric cancer in Japan, which can detect several asymptomatic GISTs [14, 24]. Finding asymptomatic tumors using established screening systems improves prognosis. In fact, the overall prognosis for patients who did not receive adjuvant therapy in this study was better than that previously reported for both 10-year OS and RFS rates (80.1% and 89.6%, respectively) [16, 23, 25]. The 10-year RFS rate of the high-risk group was significantly lower than that of the other risk groups. This result suggests that the m-NIHC had a high sensitivity (76.3%) for predicting recurrence, and this criterion is clinically important in the selection of adjuvant therapy candidates, as previously described [26].
This study is the first to validate contour maps in the Japanese population. Recurrent cases are plotted in the contour maps, which estimate the gradient recurrence risk for each patient. First, we evaluated the patients’ recurrence risk according to the contour maps, and their prognoses were evaluated. The predicted recurrence in each patient aligned well with their prognoses, with rates of recurrence within 10 years increasing with the predicted risk of recurrence. Furthermore, the actual recurrence rate was lower than the estimated recurrence rate, suggesting that the recurrence rate in Japanese patients might have more optimistic prognoses than those in other populations.
Undergoing adjuvant IM for 3 years has been established as the standard treatment for patients with a high risk of relapse, based on randomized trials [9, 17, 27]. However, the benefit for RFS seemed to decrease after the end of adjuvant therapy, with an increasing risk of relapse during this period [7,8,9]. Thus, adjuvant therapy cannot eradicate micro-residual GISTs but may merely delay the time of recurrence. Nishida et al. reported that patients with potential micrometastases are recommended to take adjuvant therapy for more than 3 years [28]. Furthermore, some clinical trials are ongoing to determine whether extending adjuvant therapy with IM over 3 years can further reduce the risk of relapse and improve OS in patients with high-risk GISTs (NCT02413736, NCT02260505 and NCT01742299). However, it remains unclear for which high-risk patients are eligible for over 3 years. According to our results, the prognosis was different in the high-risk group according to m-NIHC. And, it may be useful for determination of the candidate for more intensive therapy. Thus, patients and surgeons will be able to obtain more detailed information from the contour maps with high sensitivity to recurrence (Online Resource 1) to make personalized decisions for postoperative treatment.
Tumor rupture is a serious risk factor for recurrence. Most ruptured GISTs are associated with recurrence at follow-up, and patients with ruptured GISTs have significantly shorter RFS than that those without rupture [22, 29, 30]. In the present study, the rate of tumor rupture was 1.8% in this population. The 10 year-RFS rate of the patients with tumor rupture was quite worse than that of high risk patients without tumor rupture (Online Resource 2). Therefore, it is considered reasonable to separate ruptured GIST patients and non-ruptured patients in the contour maps.
Neoadjuvant therapy with IM has been shown by both prospective and retrospective studies to effectively decrease tumor size, thereby facilitating ease of surgery and resulting in less morbid, organ-preserving operations [31,32,33]. For these patients, conventional risk classification is not applicable because pathological features are affected by IM. In particular, the mitotic counts of the specimen after neoadjuvant therapy have been reported to decrease remarkably, and we cannot fit patients into the m-NIHC or contour maps [34]. 18F-Fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) is noninvasive and has been reported to be an effective imaging technique for assessment of malignancy and monitoring responses to IM therapy in GIST [34,35,36,37,38,39]. Proper selection of patients who require neoadjuvant therapy and risk evaluation after neoadjuvant therapy is important. However, prognostic factors such as mitotic count, exact tumor size, and tumor rupture during surgery can only be assessed postoperatively. In this regard, 18F-FDG PET/CT may aid in predicting the prognosis of neoadjuvant therapy patients. Further studies are needed to evaluate the possible role of 18F-FDG PET/CT in pathological assessment.
This study has some limitations. First, as this study was a registry study that included patients whose data were collected retrospectively, the follow-up period and way it was conducted were not standardized. Second, the very low-risk GISTs classification as defined by the m-NIHC included many incidental conditions of other serious diseases. In fact, while there were few cases of recurrence, 38 patients died during the observation period, and the 10-year OS rate was 69.4%, similar to that in the high-risk group (Online Resource 3). Of the patients who died in the very low-risk group, 92.1% were due to other diseases that were mainly the triggers for finding those small GISTs. Therefore, OS of the very low-risk GIST group does not reflect its own prognosis. Third, mutational analysis is known to be important in deciding whether to administer IM [7, 40,41,42]. However, most patients in this study did not undergo mutational analysis. Therefore, we could not evaluate the response to adjuvant therapy by genetic mutations. Additionally, since this study was excluded the high-risk patients with perioperative therapy, it might have some bias. However, this study was mainly based on patients admitted before 2012, when the standard treatment of 3-year adjuvant therapy was established, the ratio of patients with perioperative therapy was relatively low. Nevertheless, since the data were based on consecutive patients in each participating hospital, the study reflects real-world data on Japanese patients.
In conclusion, contour maps are effective in predicting GIST recurrence after primary surgery in the Japanese population. Contour maps may be useful in combination with the m-NIHC for the consideration of more individual indications for adjuvant therapy.
Data availability
The data that support the findings of this study are available upon request from the corresponding author, Tsuyoshi Takahashi. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
References
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8.
Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006;114:192–200.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83.
Teranishi R, Takahashi T, Nishida T, Kurokawa Y, Nakajima K, Koh M, et al. Plasma trough concentration of imatinib and its effect on therapeutic efficacy and adverse events in Japanese patients with GIST. Int J Clin Oncol. 2023;28:680–7.
Teranishi R, Takahashi T, Nishida T, Hirota S, Kurokawa Y, Saito T, et al. Efficacy and safety of regorafenib in Japanese patients with advanced gastrointestinal stromal tumors. Int J Clin Oncol. 2022;27:1164–72.
Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, et al. Outcome of 1000 patients with gastrointestinal stromal tumor (GIST) treated by surgery in the pre- and post-imatinib eras. Ann Surg. 2021;273:128–38.
DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PWT, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104.
Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33:4276–83.
Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–72.
Cho H, Nishida T, Takahashi T, Masuzawa T, Hirota S. Impact of the KIT/PDGFRA genotype on prognosis in imatinib-naïve Japanese patients with gastrointestinal stromal tumor. Ann Gastroenterol Surg. 2022;6:241–8.
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65.
Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Woźniak A, Limon J, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14:2018–27.
Takahashi T, Nakajima K, Nishitani A, Souma Y, Hirota S, Sawa Y, et al. An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol. 2007;12:369–74.
Yamamoto K, Tsujinaka T, Takahashi T, Sato S, Nishiguchi Y, Nakashima Y, et al. Impact of the Japanese gastric cancer screening system on treatment outcomes in gastric gastrointestinal stromal tumor (GIST): an analysis based on the GIST registry. Ann Surg Oncol. 2015;22:232–9.
Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9.
Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–74.
Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–30.
Wada N, Kurokawa Y, Nishida T, Takahashi T, Toyokawa T, Kusanagi H, et al. Subgroups of patients with very large gastrointestinal stromal tumors with distinct prognoses: a multicenter study. J Surg Oncol. 2014;109:67–70.
Sato S, Tsujinaka T, Yamamoto K, Takahashi T, Kishi K, Imamura H, et al. Primary surgery as a frontline treatment for synchronous metastatic gastrointestinal stromal tumors: an analysis of the Kinki GIST registry. Surg Today. 2016;46:1068–75.
Sato S, Tsujinaka T, Masuzawa T, Yamamoto K, Takahashi T, Yamashita Y, et al. Role of metastasectomy for recurrent/metastatic gastrointestinal stromal tumors based on an analysis of the Kinki GIST registry. Surg Today. 2017;47:58–64.
Yasui M, Tsujinaka T, Mori M, Takahashi T, Nakashima Y, Nishida T. Characteristics and prognosis of rectal gastrointestinal stromal tumors: an analysis of registry data. Surg Today. 2017;47:1188–94.
Nishida T, Cho H, Hirota S, Masuzawa T, Chiguchi G, Tsujinaka T. Clinicopathological features and prognosis of primary GISTs with tumor rupture in the real world. Ann Surg Oncol. 2018;25:1961–9.
Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour—the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37:890–6.
Shimada H. Real-world data related to the topics of 6th edition of gastric cancer treatment guidelines. Ann Gastroenterol Surg. 2021;5:730.
Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608–15.
Yanagimoto Y, Takahashi T, Muguruma K, Toyokawa T, Kusanagi H, Omori T, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015;18:426–33.
Joensuu H, Wardelmann E, Eriksson M, Reichardt A, Sundby Hall K, Schütte J, et al. KIT and PDGFRA mutations and survival of gastrointestinal stromal tumor patients treated with adjuvant imatinib in a randomized trial. Clin Cancer Res. 2023;29(17):3313–9.
Nishida T, Sato S, Ozaka M, Nakahara Y, Komatsu Y, Kondo M, et al. Long-term adjuvant therapy for high-risk gastrointestinal stromal tumors in the real world. Gastric Cancer. 2022;25:956–65.
Hølmebakk T, Hompland I, Bjerkehagen B, Stoldt S, Bruland ØS, Hall KS, et al. Recurrence-free survival after resection of gastric gastrointestinal stromal tumors classified according to a strict definition of tumor rupture: a population-based study. Ann Surg Oncol. 2018;25:1133–9.
Bischof DA, Kim Y, Dodson R, Jimenez MC, Behman R, Cocieru A, et al. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors: a multi-institutional analysis of 502 patients. JAMA Surg. 2015;150:299–306.
Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer. 2017;117:25–32.
Wang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol. 2012;19:1074–80.
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–7.
Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H, et al. 18F-fluorodeoxyglucose positron emission tomography: useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J Surg. 2005;29:1429–35.
Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9.
Van den Abbeele AD. The lessons of GIST-PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13.
Hwang SH, Jung M, Jeong YH, Jo K, Kim S, Wang J, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative (18)F-FDG PET/CT in patients with localized primary gastrointestinal stromal tumors. Cancer Metab. 2021;9:8.
Cho MH, Park CK, Park M, Kim WK, Cho A, Kim H. Clinicopathologic features and molecular characteristics of glucose metabolism contributing to 18F-fluorodeoxyglucose uptake in gastrointestinal stromal tumors. PLoS ONE. 2015;10:e0141413.
Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–64.
Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer (Oxford, England: 1990). 2004;40:689–95.
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–9.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
Open access funding provided by Osaka University.
Author information
Authors and Affiliations
Contributions
RT (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing—original draft), TT (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing—original draft), SS (Investigation, Resources, Validation, Visualization, Writing—review and editing), KS (Investigation, Resources, Validation, Visualization, Writing—review and editing), KK (Investigation, Resources, Validation, Visualization, Writing—review and editing), HH (Investigation, Resources, Validation, Visualization, Writing—review and editing), TN (Investigation, Resources, Validation, Visualization, Writing—review and editing), YK (Investigation, Resources, Validation, Visualization, Writing—review and editing), JF (Investigation, Resources, Validation, Visualization, Writing—review and editing), TN (Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Visualization, Writing—review and editing), SH (Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Visualization, Writing—review and editing), TT (Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Visualization, Writing—review and editing).
Corresponding author
Ethics declarations
Conflict of interest
Tsuyoshi Takahashi received Honoraria from Pfizer, Bayer Yakuhin, Ltd, Taiho Pharmaceutical, Nippon Kayaku and Sawai Pharmaceutical Co., Ltd. outside the submitted work, and received the payment from Taiho Pharmaceutical. Toshirou Nishida reports Honoraria from Pfizer, Taiho Pharmaceutical and Eisai Co. outside the submitted work. Other authors declare that they have no conflict of interest.
Ethics approval
The Human Ethics Review Committee of the Osaka University Graduate School of Medicine approved the protocol for this retrospective study (Approval No. 18424-2). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Consent for publication
Consent for publication was obtained from all individuals whose data appears in the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teranishi, R., Takahashi, T., Sato, S. et al. The impact of contour maps on estimating the risk of gastrointestinal stromal tumor recurrence: indications for adjuvant therapy: an analysis of the Kinki GIST registry. Gastric Cancer 27, 355–365 (2024). https://doi.org/10.1007/s10120-023-01444-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01444-8