Abstract
Background
Prophylactic total gastrectomy (PTG) remains the only means of preventing gastric cancer for people with genetic mutations predisposing to Hereditary Diffuse Gastric Cancer (HDGC), mainly in the CDH1 gene. The small but growing cohort of people undergoing PTG at a young age are expected to have a life-expectancy close to the general population, however, knowledge of the long-term effects of, and monitoring requirements after, PTG is limited. This study aims to define the standard of care for follow-up after PTG.
Methods
Through a combination of literature review and two-round Delphi consensus of major HDGC/PTG units and physicians, and patient advocates, we produced a set of recommendations for follow-up after PTG.
Results
There were 42 first round, and 62 second round, responses from clinicians, allied health professionals and patient advocates. The guidelines include recommendations for timing of assessments and specialties involved in providing follow-up, micronutrient supplementation and monitoring, bone health and the provision of written information.
Conclusion
While the evidence supporting the guidelines is limited, expert consensus provides a framework to best manage people following PTG, and could support the collection of information on the long-term effects of PTG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary diffuse gastric cancer (HDGC) is a rare, heritable condition caused by germline mutations, mainly in the CDH1 cancer susceptibility gene [1]. Estimated prevalence in the population is 1/5000 [2]. Carriers have a substantially increased risk of diffuse gastric cancer and lobular breast cancer [1]. International guidelines recommend prophylactic total gastrectomy (PTG) prior to the development of advanced gastric cancer, often between the ages of 20 and 40 years. We have no clear understanding of the long-term effects of PTG. Limited, historical published evidence is available for the medium-term effects of gastric resection on ulcer disease and cancer. However, these studies do not reflect the patient population undergoing PTG.
A range of guidelines exist for follow-up after gastrectomy for cancer [3, 4], they do not touch on the range of challenges experienced by the young population affected by HDGC, and we believe this group will benefit from a tailored set of guidelines.
While it is recognised that long-term follow-up is advisable, the nature of this follow-up has not been defined. Experience and expertise are available in international units offering PTG and follow-up. It is important to recognise the similarities in post-operative anatomy and so theoretically late effects between PTG and Roux-en-Y gastric bypass (RYGB) for obesity. Detailed guidelines exist for follow-up after RYGB in many countries, providing a model to guide follow-up after PTG.
Collation of current practice, a review of the literature and a Delphi consensus of experts and patient representatives were used to create a set of guidelines to guide patient care [5, 6]. Future review of these guidelines could include a review of the outcomes of the investigations detailed below and potentially allow for more evidence-based practice.
Materials and methods
Detailed methodology is available in the supplementary material. In brief, the process used to create the guidelines involved:
-
1.
Recruitment of a steering committee from a multidisciplinary group of experts in academic settings with variation in geographic region and areas of expertise.
-
2.
Literature review (using GRADE levels of evidence [7]) and collation of current standards from international units.
-
3.
Discussion of key priorities and questions in the steering committee.
-
4.
Creation of first-round guideline statements.
-
5.
Review by the steering group.
-
6.
Open Delphi consensus round 1 by a confidential vote of study members made up of patient representatives and clinicians experienced in the care of patients with HDGC (identified through membership of the International Gastric Cancer Linkage Consortium (IGCLC)).
-
7.
Review of consensus results and modification of statements by the steering committee.
-
8.
Open Delphi consensus round 2 by confidential vote.
-
9.
Review of consensus results and agreement of final statements by the steering committee.
Interpretation of these guidelines must acknowledge the very low level of evidence supporting almost all the statements. To await the development of evidence would consign the current generation of those who have undergone PTG to potentially inadequate care. The study group has taken a pragmatic approach to the recommendation of statements, considering the impact of the recommended action on the person receiving care as well as the health system providing that care. Low-intensity interventions (e.g. clinical review) are given greater weight than higher-intensity interventions (e.g. DXA scan) despite a similar level of agreement between study respondents.
The implementation of these guidelines may lead to the collation of long-term data and allow the formulation of more evidence-based recommendations in future years. These guidelines will be reviewed at the IGCLC meeting (Porto, 2024) and a consensus agreed upon an appropriate interval for audit of findings and revision of guidelines.
The recommendations are published in accordance with the AGREE reporting checklist [8].
Results
Review of the published literature revealed no directly relevant long-term case series or experimental studies. These guidelines were written with regard to current practice, basic scientific principles and the limited available literature.
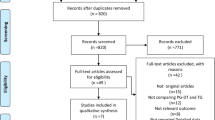
Forty-two participants voted in the first round of the consensus, and sixty-two in the second round (Tables 1 and 2). The first-round statements and detailed results of both Delphi rounds are available in the supplementary material.
Consensus Statements—all statements, recommendations and evidence levels are detailed in Table 3.
Statement 1: What specialties should be available to the post-PTG multi-disciplinary team?
Post-PTG care requires a multi-disciplinary team with the lead specialty usually surgery or gastroenterology. Core specialties recommended for routine follow-up were surgery, gastroenterology, dietetics and specialist nursing. A number of specialties likely to be helpful to people during the course of their lives, including psychologist, geneticist, internal medicine physician, pharmacist, exercise therapist and fertility clinician were also identified. While not specifically relevant to PTG follow-up, female patients should receive appropriate breast surveillance.
Patient representatives strongly suggested that pharmacists and exercise therapists be involved in care after PTG. Drug absorption may be altered by the absence of gastric acidity and rapid transition of ingested nutrients to the small bowel[1] and specific formulations (e.g. modified release or enteric coated) of some drugs may be inadequately absorbed. Anecdotal reports of unplanned pregnancies after PTG despite regular oral contraception may be due to altered absorption. Specialist pharmacist review of both prescribed and over-the-counter medications may obviate some of this risk.
Exercise or physio-therapists may help with return to function post-surgery, as well as with physical fitness and maintenance of lean mass in the longer term.
Statement 2: What is the recommended follow-up interval for patients after PTG?
There is a general consensus that people should be offered long-term follow-up after PTG, although evidence for frequency and duration is lacking. Follow-up can be divided into early (i.e. before full recovery from surgery) and long-term (to monitor for late effects of surgery). The boundary between the two is fluid, but best regarded as being the point at which the person’s weight is stable, which is likely to be 1–2 years, and they have returned to normal activities, which often occurs much sooner. Oftentimes, patients will be fully able to return to work and normal activities while experiencing continued slow weight loss.
Statement 3: What factors should be assessed at routine follow-up?
A core set of factors to be addressed at routine follow-up was identified by discussion amongst the steering committee and from free-text responses to the first Delphi round. There was strong consensus that this should include dietitian review, measurement of weight, review of GI symptoms, blood tests (detailed below), discussion of any other cancer screening (e.g. breast) [1], and identification of whether pharmacist, psychologist or obstetrician review would be appropriate.
Statement 4: What routine supplements, if any, should be recommended after PTG?
After PTG, a degree of micronutrient malabsorption is assumed due to reduced proximal gut exposure and fat malabsorption. Iron and vitamin B12 deficiencies are well described in patients after gastrectomy, and supplementation and monitoring are supported by observational evidence. Micronutrients of particular interest are discussed below, with the rationale for supplementation and monitoring discussed further.
Fat-soluble vitamins
There is a theoretical risk of fat-soluble vitamin malabsorption following PTG, due to the Roux loop removing the normal stimulus for bilio-pancreatic secretions.
There are conflicting results on the prevalence of vitamin A deficiency after gastrectomy [9, 10]. It is crucial to note the teratogenic risk of over-supplementation of vitamin A during pregnancy [9].
Several small cohort studies report up to 20% incidence of vitamin E deficiency after gastrectomy, with up to half suffering neurological sequelae [10,11,12]. The risk of vitamin E deficiency appears to increase with time after gastrectomy, and in one series became clinically significant at approximately 4 years [12].
Vitamin D and calcium deficiency are discussed in the bone health section. It is worth noting that there is some limited evidence of improved gastrointestinal calcium absorption when administered in the citrate, rather than the carbonate, form [13, 14].
Vitamin B12
Vitamin B12 deficiency is a well-established consequence of total gastrectomy and all people after PTG should receive routine vitamin B12 supplementation [15]. Standard practice in many units is intramuscular vitamin B12, while high-dose oral formulations (1 mg/day) are also in use and have been shown to be effective [16, 17]. Sublingual, transdermal and intranasal routes have also been described [18,19,20].
Thiamine/vitamin B1
One study reported that 5 out of 32 patients after total gastrectomy suffered thiamine deficiency [21]. Another case series reported on 17 post gastrectomy patients suffering neurologically significant thiamine deficiency without routine supplementation [22]. Thiamine deficiency, and consequent Wernicke-Korsakoff syndrome should be considered in all people post PTG with significant weight loss or prolonged vomiting.
Iron
Iron deficiency has been found in up to 66.7% of women, and 34.7% of men after gastrectomy [17, 23, 24]. Iron is absorbed in the proximal gut in acidic conditions, hence the risk of deficiency after gastrectomy. Recent studies suggest alternate day iron supplements to be more effective than daily iron supplementation [25] and there is evidence suggesting that polymaltose iron supplements are associated with fewer side effects [26]. Intravenous iron supplementation is also a valid option. Iron supplementation was a topic of differing opinion between the two patient representatives in the steering committee; one was very keen to recommend routine higher dose supplements to avoid the unpleasant symptoms of iron deficiency, whereas the other felt that the unpleasant side effects of supplements should be avoided if possible. This is an area where post-operative follow-up may be tailored to the individual, considering diet, gender, age and past medical history.
Zinc, selenium, copper
Zinc and copper are absorbed in the duodenum and proximal jejunum. Zinc deficiency has been reported in up to 51%, and selenium deficiency in up to 39% of patients after oesophagectomy or gastrectomy [27]. There are no case series examining copper deficiency, however, as copper and zinc absorption are closely linked, they should be considered together. Of note, replacement of copper and zinc when deficient should be done together (even when only one is deficient) to prevent dangerous shifts in copper and zinc levels.
Synopsis of statement 4
-
The consensus is to recommend routine supplementation with a multi-vitamin and mineral after PTG, as well as vitamins D, B12 and calcium to higher levels than in basic multivitamin formulations. Routine micronutrient supplementation was a topic of disagreement between the core study group, with several members keen to stress the lack of evidence and the burden of cost.
-
Replacement of deficient micronutrients should be guided by monitoring.
-
Iron deficiency is common and intravenous replacement is an important option. Routine iron supplementation can be offered on a case-by-case basis.
-
Management of micronutrient supplementation, particularly folate and vitamin A, before and during pregnancy should be guided by a specialist obstetrics service.
Statement 5: Post-operative blood tests
There is no evidence that routine monitoring is of utility in the prevention of clinically significant deficiencies, however, it is standard practice in many units, and recommended after bariatric surgery [28, 29]. Assays were regarded as “Routine” or “Optional”, considering the likelihood of deficiency and the availability and cost of assays.
We recommend all patients initially receive annual tests as detailed in Table 3. Optional tests can be included at the discretion of the unit and used as a guide to investigate symptomatic deficiencies. As many people will survive decades after their surgery, a life-long index of suspicion must be maintained for micronutrient deficiency. Sufficient pre-operative stores of some micronutrients may exist to prevent clinically relevant deficiencies from developing for years after PTG.
Optional tests are conditionally recommended on a case-by-case basis, recognising the limited evidence of their utility and the high cost and limited availability of the specific tests.
Statement 6: After PTG, what strategies should be pursued to prevent and identify reduced bone mineral density (BMD)?
Several mechanisms (calcium/vitamin D malabsorption, loss of load-bearing weight, altered gut hormone metabolism, and whether pathological CDH1 impacts on bone health) could theoretically result in reduced BMD after PTG. Measuring BMD is invasive and expensive, and there is a need to balance the financial and personal costs of investigation with the possible benefit to current and future generations. A range of experimental and observational studies indicate a possible risk of reduced BMD after gastrectomy and have been considered in these guidelines.
One meta-analysis including 1206 patients identified a pooled risk of osteoporosis after gastrectomy of 36% [30], with risk factors for post-gastrectomy osteoporosis include female gender and weight loss [31, 32]. A Korean study including 133,179 subjects identified a two-fold increase in osteoporotic fracture risk after gastrectomy compared to matched controls [33], and a smaller but well-designed cohort study identified the odds ratio for developing osteoporosis ten years post gastrectomy was 8.69 vs unoperated matched controls [34]. Two observational studies reported a risk of post-gastrectomy pathological fracture of 25% at 5 years and 37% at 6 years [31, 35].
Three smaller studies compared pre- and post-operative bone mineral density with DXA scans [36,37,38] and identified significant BMD reductions at 1-year post gastrectomy, and a study using bone biopsies showed increased bone turnover post-gastrectomy [39].
Several studies identified a high incidence of vitamin D deficiency (up to 95% at 5 years post-op) and hyperparathyroidism (up to 50% at 5 years post-op) [30, 40,41,42,43]. Treatment with high dose-vitamin D resulted in the normalisation of vitamin D and PTH levels [43]. This has not been extended to investigate the effect of vitamin D treatment on BMD after gastrectomy.
The American Society for Metabolic and Bariatric Surgery guidelines for follow-up after bariatric surgery recommend all patients receive 1200–1500 mg/day of elemental calcium and 3000 IU/day of Vitamin D supplementation, and that consideration is given to DXA measurement of BMD at 2 years post-operatively [28]. It would be reasonable to consider applying these guidelines to the care of patients undergoing gastrectomy, with the proviso that obesity is likely to have an independent effect on BMD.
The guidelines published here strongly recommend vitamin D measurement and correction before PTG if time allows, and routine post-operative calcium and vitamin D supplementation and monitoring. While the evidence is limited, the cost is minimal and the potential for prevention of later osteoporosis significant.
The topic of monitoring bone health proved controversial both within the core study group and across the respondents to the Delphi consensus. When asked to state a preference for one of two options including routine post-PTG DXA scanning (Table 4), versus no routine DXA, the majority of respondents (44) were in favour of routine pre- and 3-year post-operative DXA in addition to DXA at age 50 or menopause. However, when considering the cost in terms of resources and patient time against the very limited evidence, the study group felt it more appropriate to make a conditional recommendation for DXA scanning. Individual units are advised to consider monitoring BMD, on a case-by-case basis and to consider routine DXA in patients over 50 or at the age of menopause. Several units routinely measure BMD after PTG, and it is hoped that when this data is published a more evidence-based approach to monitoring bone health will be recommended.
In people who develop reduced BMD after PTG, it is recommended that management is guided by a specialist in metabolic bone diseases where available.
Statement 7: What written information or education should be provided to patients following PTG?
The provision of written information to patients varies between units. Patient advocates strongly supported the provision of written information to patients about their operation and recovery. We sought to clarify the most important aspects of the written information, considering local policies and culture. We have not sought to define more than a general outline of this area, however, we recognise that a significant amount of the published patient information will apply across all units and there is potential for the development of a central resource of information templates.
Conclusions
This is the first set of published guidelines for the follow-up of people after PTG and defines a set of standards for units to consider implementing after surgery. Although there is limited evidence supporting many of the statements, the steering committee reached a consensus by collating international expertise and patient opinions to identify sensible and pragmatic standards of care. The aim was to consider the uncertainty of long-term complications of PTG alongside the risks of medicalising people who have recovered from surgery, and the resource implications of life-long review and tests.
We recognise that there will be barriers to the implementation of these guidelines. Provision of follow-up appointments (in person or remotely), blood tests and DXA scans require funding and other resources. They also require expertise in the management of post-operative patients, and particularly the challenges of managing nutritional and gastrointestinal sequelae of PTG. While we have attempted to avoid proscriptive recommendations for expensive or difficult-to-access tests and supplements, the availability of funding and expertise could limit the full implementation of these guidelines in some settings.
It is envisaged that these guidelines will be available to support units in the development of local protocols for the long-term follow-up of their PTG patients. Their utility and challenges will be discussed at the next IGCLC congress (Porto, 2024). While most of the guidelines require no further tools to implement, we acknowledge that patient education literature will need to be developed in response to our recommendations. While some of this work will have to reflect local medical practice and culture, there is potential for a future project to develop centrally accessible post-PTG educational literature.
Future directions
A central theme of discussions while writing these guidelines was the need to develop an evidence base for PTG follow-up. The questions we have sought to answer could form the basis of an interdisciplinary approach to post-PTG research. Indeed, the data collected by units following these guidelines could guide future projects and feed into revisions of these guidelines. Areas of particular interest include estimating and managing the risk of post-PTG osteoporosis/osteopaenia; prevention, identification and treatment of micronutrient deficiencies; identification and management of GI complications of PTG (e.g. dumping syndrome, pancreatic insufficiency, reflux); patient information literature; and templates or guides for follow-up appointments.
We acknowledge that despite our best efforts to contact and involve as many international HDGC units as possible in this study, we may not have reached many others. This can be rectified when the guidelines are revised at the IGCLC meeting in 2024.
References
Blair VR, McLeod M, Carneiro F, Coit DG, D’Addario JL, van Dieren JM, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21(8):e386–97. https://doi.org/10.1016/S1470-2045(20)30219-9 (Epub 2020/08/08).
Bar-Mashiah A, Soper ER, Cullina S, Belbin GM, Kenny EE, Lucas AL, et al. CDH1 pathogenic variants and cancer risk in an unselected patient population. Fam Cancer. 2021. https://doi.org/10.1007/s10689-021-00257-x (Epub 2021/04/23).
Nilsson M. Postgastrectomy follow-up in the West: evidence base, guidelines, and daily practice. Gastric Cancer. 2017;20(Suppl 1):135–40. https://doi.org/10.1007/s10120-016-0654-9 (Epub 2016/10/09).
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57-63. https://doi.org/10.1093/annonc/mdt344 (Epub 2013/10/23).
Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):1–88.
Dalkey N, Helmer O. An Experimental Application of the DELPHI Method to the Use of Experts. Manag Sci. 1963;9(3):458–67.
Schunemann H, Brozek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. Updated October 2013.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. CMAJ. 2010;182:E839–42.
Husemann B, Groitl H, Zirngibl H. Metabolic and surgical aspects of total gastrectomy [German]. Munchener Medizinische Wochenschrift. 1978;120(16):561–4.
Rino Y, Oshima T, Yoshikawa T. Changes in fat-soluble vitamin levels after gastrectomy for gastric cancer. Surg Today. 2017;47(2):145–50.
Rino Y, Yukawa N, Sato T, Yamamoto N, Tamagawa H, Hasegawa S, et al. Vitamin E deficiency begins within 6 months after gastrectomy for gastric cancer. World J Surg. 2014;38(8):2065–9. https://doi.org/10.1007/s00268-014-2515-1 (Epub 2014/03/29).
Ueda N, Suzuki Y, Rino Y, Takahashi T, Imada T, Takanashi Y, et al. Correlation between neurological dysfunction with vitamin E deficiency and gastrectomy. J Neurol Sci. 2009;287(1–2):216–20. https://doi.org/10.1016/j.jns.2009.07.020 (Epub 2009/08/28).
Sakhaee K, Bhuket T, Adams-Huet B, Rao DS. Meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate. Am J Ther. 1999;6(6):313–21. https://doi.org/10.1097/00045391-199911000-00005 (Epub 2001/05/01).
Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22(3):286–96. https://doi.org/10.1177/0115426507022003286 (Epub 2007/05/18).
Hu Y, Kim HI, Hyung WJ, Song KJ, Lee JH, Kim YM, et al. Vitamin B(12) deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg. 2013;258(6):970–5. https://doi.org/10.1097/sla.0000000000000214 (Epub 2013/10/08).
Moleiro J, Mao de Ferro S, Ferreira S, Serrano M, Silveira M, Dias Pereira A. Efficacy of long-term oral vitamin B12 supplementation after total gastrectomy: results from a prospective study. Port. 2018;25(3):117–22. https://doi.org/10.1159/000481860.
Kim J. Long-term trends in hematological and nutritional status after gastrectomy for gastric cancer. United Eur Gastroenterol J. 2017;5(5 Supplement 1):833. https://doi.org/10.1177/2050640617725676.
Bensky MJ, Ayalon-Dangur I, Ayalon-Dangur R, Naamany E, Gafter-Gvili A, Koren G, et al. Comparison of sublingual vs. intramuscular administration of vitamin B12 for the treatment of patients with vitamin B12 deficiency. Drug Deliv Transl Res. 2019;9(3):625–30. https://doi.org/10.1007/s13346-018-00613-y (Epub 2019/01/12).
Slot WB, Merkus FW, Van Deventer SJ, Tytgat GN. Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology. 1997;113(2):430–3. https://doi.org/10.1053/gast.1997.v113.pm9247460 (Epub 1997/08/01).
Saurabh S, Gao Y, Maduka S, Smith L, Lasley R, Singh N. Is transdermal multivitamin patch effective in gastric bypass patients? Obes Surg. 2019;29(12):3818–23. https://doi.org/10.1007/s11695-019-04070-5 (Epub 2019/07/16).
Iwase K, Higaki J, Yoon HE, Mikata S, Miyazaki M, Kamiike W. Reduced thiamine (vitamin B1) levels following gastrectomy for gastric cancer. Gastric Cancer. 2002;5(2):77–82. https://doi.org/10.1007/s101200200013 (Epub 2002/07/12).
Koike H, Misu K, Hattori N, Ito S, Ichimura M, Ito H, et al. Postgastrectomy polyneuropathy with thiamine deficiency. J Neurol Neurosurg Psychiatry. 2001;71(3):357–62. https://doi.org/10.1136/jnnp.71.3.357 (Epub 2001/08/21).
Kim JH, Bae YJ, Jun KH, Chin HM. The prevalence and clinical significance of postgastrectomy anemia in patients with early-stage gastric cancer: a retrospective cohort study. Int J Surg. 2018;52:61–6. https://doi.org/10.1016/j.ijsu.2018.02.037.
Lim CH, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, et al. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;18(42):6114–9. https://doi.org/10.3748/wjg.v18.i42.6114.
Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–9. https://doi.org/10.3324/haematol.2019.220830 (Epub 2019/08/16).
Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3(1):12–33. https://doi.org/10.3390/pharmaceutics3010012 (Epub 2011/01/01).
Kidd A, Macharg F, Westmancoat E, Preston S. The annual risk of post-operative vitamin & mineral deficiencies following oesophageal and gastric cancer surgery. Dis Esophagus. 2014;27:117A.
Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—executive summary. Endocr Pract. 2019;25(12):1346–59. https://doi.org/10.4158/gl-2019-0406 (Epub 2019/11/05).
O’Kane M, Parretti HM, Pinkney J, Welbourn R, Hughes CA, Mok J, et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes Rev. 2020;21(11):e13087. https://doi.org/10.1111/obr.13087 (Epub 2020/08/04).
Oh HJ, Yoon BH, Ha YC, Suh DC, Lee SM, Koo KH, et al. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: a meta-analysis. Osteoporos Int. 2020;31(2):267–75. https://doi.org/10.1007/s00198-019-05220-2 (Epub 2019/11/30).
Oh HJ, Lim CH, Yoon BH, Yoon SB, Baeg MK, Kim WC, et al. Fracture after gastrectomy for gastric cancer: a long-term follow-up observational study. Eur J Cancer. 2017;72:28–36. https://doi.org/10.1016/j.ejca.2016.11.023.
Yoo SH, Lee JA, Kang SY, Kim YS, Sunwoo S, Kim BS, et al. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer. 2018;21(4):720–7. https://doi.org/10.1007/s10120-017-0777-7.
Shin DW, Suh B, Lim H, Suh YS, Choi YJ, Jeong SM, et al. Increased risk of osteoporotic fracture in postgastrectomy gastric cancer survivors compared with matched controls: a nationwide cohort study in Korea. Am J Gastroenterol. 2019;114(11):1735–43. https://doi.org/10.14309/ajg.0000000000000436 (Epub 2019/10/29).
Park KB, Jeon CH, Lee HH, Chin H, Song KY. Prediction of risk of osteoporosis after gastrectomy for gastric cancer. BJS Open. 2021. https://doi.org/10.1093/bjsopen/zrab123 (Epub 2021/12/22).
Seo GH, Kang HY, Choe EK. Osteoporosis and fracture after gastrectomy for stomach cancer. Medicine (United States). 2018. https://doi.org/10.1097/MD.0000000000010532.
Noh HM, Yoo JH, Jeong JY, Park YS. Bone mineral density after treatment for gastric cancer. Medicine (United States). 2018. https://doi.org/10.1097/MD.0000000000009582.
Atsumi Y, Rino Y, Wada H, Kitani Y, Ozawa Y, Aoyama T, et al. Changes in bone metabolism after gastric cancer surgery in male patients: a prospective observational study. Gastric Cancer. 2019;22(1):237–43. https://doi.org/10.1007/s10120-018-0835-9 (Epub 2018/05/12).
Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY, et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone. 2008;42(1):61–7. https://doi.org/10.1016/j.bone.2007.08.027 (Epub 2007/10/19).
Krause M, Keller J, Beil B, van Driel I, Zustin J, Barvencik F, et al. Calcium gluconate supplementation is effective to balance calcium homeostasis in patients with gastrectomy. Osteoporos Int. 2015;26(3):987–95. https://doi.org/10.1007/s00198-014-2965-1.
Wetscher G, Redmond E, Watfah C, Perdikis G, Gadenstatter M, Pointner R. Bone disorders following total gastrectomy. Dig Dis Sci. 1994;39(12):2511–5. https://doi.org/10.1007/BF02087683.
Bisballe S, Eriksen EF, Melsen F, Mosekilde L, Sorensen OH, Hessov I. Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodelling, vitamin D metabolites, and bone histomorphometry. Gut. 1991;32(11):1303–7.
Lee YK, Kim DY, Ha YC, Lee Y, Byun DW, Chung HY, et al. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: a meta-analysis. Bone Reports. 2020;Conference: ECTS Congress 2020. 13(Supplement). https://doi.org/10.1016/j.bonr.2020.100427.
Climent M, Pera M, Aymar I, Ramón JM, Grande L, Nogués X. Bone health in long-term gastric cancer survivors: a prospective study of high-dose vitamin D supplementation using an easy administration scheme. J Bone Miner Metab. 2018;36(4):462–9. https://doi.org/10.1007/s00774-017-0856-1 (Epub 2017/08/03).
Acknowledgements
The authors would like to thank the several contributors who wished to remain anonymous, as well as our patients and colleagues who have helped to illuminate the questions we seek to answer in this study. List of study contributors (the Life after Prophylactic Total Gastrectomy study group) in addition to the named authors: Julie Angel LICSW (NIH, USA), Banu Arun MD (University of Texas MD Anderson Cancer Center), Manuela Baptista MD (Centro Hospitalar Universitário de São João, Portugal), Grant Beban MB ChB FRACS (Auckland City Hospital, New Zealand), Ernst Jan Spillenaar Bilgen MD PhD (Rijnstate Hospital, Netherlands), Alex Boussioutas MBBS PhD FRACP AGAF (The Alfred, Monash University, Australia), Simone Busija (Patient advocate), Carlos Caldas MD (University of Cambridge, UK), Fatima Carneiro MD PhD (Ipatimup, Porto, Portugal), Annemieke Cats MD PhD (Netherlands Cancer Institute), Maureen Connolly RN (NIH, USA), Johanna D'Addario (Patient advocate), Massimiliano di Pietro MD (University of Cambridge), Cuong Duong MBBS PhD FRACS (Peter MacCallum Cancer Centre, Melbourne, Australia), Naheed Farooq FRCS (Greater Manchester Oesophagogastric service, UK), Rebecca Fitzgerald MD FMedSci (Hutchison-MRC Research Centre, University of Cambridge, UK), Claire Forde MB BCh BAO (Manchester Centre for Genomic Medicine, UK), Lauren Gamble MD (NIH, USA), Kimberley Gamet FHGSA (Genetic Health Service New Zealand), Irene Gullo MD PhD (Ipatimup, Centro Hospitalar Universitário de São João, Portugal), Trevor Hamilton MD MSc (University of British Columbia, Canada), Nicoline Hoogerbrugge MD PhD (Radboud University Medical Center, Nijmegen, Netherlands), Shannon Hopkins MS RD CDN (Memorial Sloan Kettering Cancer Center), Bryson W Katona MD PhD (University of Pennsylvania, USA), Sonia Kupfer MD (University of Chicago, USA), Madison LaRose APRN MSN FNP-C (University of Texas MD Anderson Cancer Center), Jeremie H. Lefevre MD PhD (Hopital Saint-Antoine, Sorbonne Université, France), Rachael Lopez MPH RD CSO (NIH, USA), Julie Moskowitz MS CGC (University of Texas MD Anderson Cancer Center), Kathryn Munder MS RD CSO CNSC (University of Texas MD Anderson Cancer Center), Enrique Norero MD (Hospital Sotero del Rio, Chile), Yann Parc MD PhD (Hopital Saint-Antoine, Sorbonne, France), Karyn Paringatai PhD (University of Otago, New Zealand), Susan Parry FRACP (New Zealand Familial GI Cancer Service), Suraj Rajasimhan PharmD (NIH, USA), Amanda Rhodes Psy.D (NIH, USA), Ross Roberts MB ChB FRACS (Christchurch Hospital, New Zealand), Kasmintan Schrader MBBS, FRCPC, PhD, DABMG (BC Cancer, Vancouver, Canada), Carol Semrad MD (University of Chicago, USA), Ben Smith RN Dip He, BA Hons (Cambridge University Hospitals, UK), Claire Smith MDietSt (Patient Advocate, Australia), Fabiana Sousa MD (Centro Hospitalar Universitário de São João, Portugal), Elena Stoffel MD MPH (University of Michigan, USA), Nicola Sunderland RD (Cambridge University Hospitals, UK), Magali Svrcek MD PhD (Sorbonne University, AP-HP, France), Marc Tischkowitz MD PhD (University of Cambridge, UK), Jolanda van Dieren Md PhD (Netherlands Cancer Institute), Bart Witteman MD PhD (Rijnstate Hospital, Netherlands), Yanghee Woo MD (City of Hope Hospital, Duarte, USA), Sam Yoon MD (Memorial Sloan Kettering Cancer Center).
Funding
This study was completed without dedicated funding.
Author information
Authors and Affiliations
Consortia
Contributions
The initial concept of the study was a collaborative effort of a group of clinicians in the IGCLC consortium, including the named authors. GR and PK designed the study, conducted the literature review and oversaw data collection and distribution. GR wrote the initial draft of the paper. All named authors critically reviewed the results of the Delphi rounds and contributed to the evolution of the statements published for review by the study contributors, as well as contributing their particular expertise to the guidelines. All authors had editorial oversight and review of the final draft of the paper. The named contributors in the LAP-TG study group contributed to at least one Delphi round, including the provision of free text advice, and explicitly gave their consent to be named in the publication as study contributor.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Stanich receives research support from Emtora Biosciences, Freenome Holdings Inc, Janssen Pharmaceuticals Inc., Pfizer Inc. and the PTEN Research Foundation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the LAP-TG Study Group are listed in acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberts, G., Benusiglio, P.R., Bisseling, T. et al. International Delphi consensus guidelines for follow-up after prophylactic total gastrectomy: the Life after Prophylactic Total Gastrectomy (LAP-TG) study. Gastric Cancer 25, 1094–1104 (2022). https://doi.org/10.1007/s10120-022-01318-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01318-5