Abstract
Background
The nationwide registry of the Japanese Gastric Cancer Association collected data of surgically resected cases of gastric cancer between 2001 and 2013. These retrospective analyses aimed to delineate tumor characteristics, surgical history, and survival distribution.
Methods
Data from 254,706 patients with primary gastric cancer were included. The 5-year survival rates were calculated for various subsets of prognostic factors.
Results
The number of patients over 70 years old increased from 2001 to 2013. The frequency with which laparoscopic gastrectomy was opted for increased dramatically (from 3.5 to 40.8%) in 13 years. We focused on the patients registered between 2010 and 2013, for whom data collection was based on the 3rd edition of the Japanese classification and guidelines. Five-year overall survival (OS) rate among 92,305 patients with resected tumors was 70.6%. The 5-year OS rates of patients with pathological stage IA, IB, IIA, IIB, IIIA, IIIB, IIIC, and IV disease were 89.6%, 83.2%, 77.6%, 68.1%, 59.3%, 45.6%, 29.9%, and 14.0%, respectively.
Conclusion
Our detailed analysis highlights the historical changes in outcomes of surgically treated gastric malignancies in Japan, and provides robust dataset for future analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A national gastric cancer registration project [1] initiated in 1968 by the Japanese Research Society for Gastric Cancer, was rebooted in 2001 by the Japanese Gastric Cancer Association (JGCA) after a 10-year period of inactivity [2, 3]. Katai et al. reported the outcomes for registered gastric cancer patients who had a surgical intervention between 2001 and 2007 [4]. In 2010, the JGCA simultaneously revised the Japanese Classification of Gastric Carcinoma (JC) [5] and the Japanese Gastric Cancer Treatment Guidelines (JGL) [6]. The concepts of both integrated systems were individuated as follows [7]: The JC provides the basic rules to describe the status of a tumor and the evaluation of treatment outcome. The JGL defines the treatment methods and clarifies the indications for and details of each treatment. The descriptions of tumor status in the JC (T/N/M categories, stage grouping, etc.), and those in the UICC/TNM 7th edition [8] were identical; this allowed the Japanese experience to be expressed using international terminology. We herein overviewed the nationwide gastric cancer registry from 2001 to 2013. We particularly focused on cases between 2010 and 2013, which were described according to the 3rd JC and JGL with regard to tumor characteristics, surgical history, and survival distribution.
The aim of this retrospective study was to update the tumor characteristics, surgical details, and survival distribution of gastric cancer patients who underwent surgical resection between 2010 and 2013. We compared these results to previous data gathered from patients between 2001 and 2007 [4], and overviewed the trends in the treatment results from 2001 to 2013 in Japanese hospitals. This baseline information can be used in various medical communities around the world for design of future research or clinical trials.
Patients and methods
The Registration Committee of the JGCA requested hospitals affiliated with JGCA members to enroll voluntarily consecutive patients with primary gastric cancer who underwent surgical treatment from January 2001 through December 2013, which were reported annually on the home page of JGCA [9]. Hospitals first obtained a copy of the database and ensured that they met the JGCA requirements. Five years after the patients had undergone surgery, hospitals sent the anonymized data to the JGCA data center located at Niigata University Medical and Dental Hospital. The retrospective collection of data was related to 53 items, including surgical procedures, pathological diagnosis, and prognosis. The data analyzed by the JGCA Registration Committee following earlier procedures [2]. From 2001 to 2009, definitions and item reporting were based on the JGCA JC 2nd English edition [10] and the Union for International Cancer Control TNM Classification of Malignant Tumors, 5th edition [11]. From 2010 to 2013, the data were based on the JGCA JC 3rd edition [5] and JGL 3rd edition [6].
The following data were calculated: total patients; patients lost to follow-up within 5 years; survival rates by year; standard error of 5 year survival; 5-year survivors; death from gastric cancer, other cancer, other disease, and unknown cause; and recurrence site, such as local, lymph node metastasis, peritoneal metastasis, liver metastasis, and unknown site. The 5-year OS rates and disease-specific survival (DSS) rates for various subsets of prognostic factors were derived from Kaplan–Meier analysis. In the DSS analysis, deaths from the primary disease during the 5-year postoperative period were counted as events. Differences in survival curves and P values were determined using the log-rank test. The nationwide registration program was approved by the Ethics Committee of the JGCA. Every hospital discloses information about the nationwide registry of the JGCA to the patients. Participating patients were included on the study unless they informed study leads that they were unwilling to participate.
Results
Between 2001 and 2013, 277,451 patients with primary gastric carcinoma were enrolled in the JGCA nationwide registry. Of these, 270,625 underwent surgery and 256,691 underwent gastric resection. After exclusion of 1985 cases with missing data, we analyzed data from the remaining 254,706 patients who underwent gastric resection. The age distribution of patients and the historical surgical procedures are shown in Figs. 1 and 2. The proportion of patients over 70 years old increased during this period. Furthermore, there was a dramatic increase in the frequency with which laparoscopic gastrectomy was performed (rising from 3.5 to 40.8% in 13 years).
We focused on the patients registered between 2010 and 2013 according to the JGCA JC 3rd edition [5] and JGL 3rd edition [6]. The patient demographics of 92,305 cases in this period are summarized in Table 1. The fraction of patients above 80 years of age was 15.2%. The male to female ratio was 2.2:1. The proportions of location, macroscopic type, and histological type of tumor were almost the same as those reported between 2001 and 2007 [4]. Around half of all patients had early gastric cancer (T1) according to pathology reports. Among the early gastric cancer patients, the proportion individuals with surgically treated mucosal cancer gradually decreased from 27% (3071/11,194) in 2001 to 23% (4847/21,527) in 2010, and to 21% (4739/22,968) in 2013. The mucosa to submucosa ratio was 0.8:1, and 40% of patients had nodal involvement. According to JGCA JC 3rd edition [5], the percentages of pathological N1, N2, N3a, and N3b were 13.5%, 11.2%, 9.6%, and 6.1%, respectively. Comparing these data with those from 2001 to 2007 [4], we observed a similar proportion (about 40%) of patients with positive nodal metastasis (pN+). Peritoneal washing cytology was carried out in 53.5% of patients, 83.1% of patients with pT4a (serosa exposed) disease, and 84.4% of patients with pT4b (serosa infiltrating) disease. Cytology was positive in 21.0% of patients with pT4a disease, 24.6% of patients with pT4b disease, and 5.2% of patients in the overall population for whom intraoperative peritoneal lavage cytology was performed. Synchronous liver metastases were detected in 1.6% of patients, and peritoneal seeding was identified in 4.2% of patients, which slightly decreased than in patients of the 2001–2007 study (2.2% and 5.3%, respectively). These decreases might be attributed to patients undergoing gastrectomy with curative intent, as recommended by the guidelines. The majority (53%) of the patients had pathological stage I disease; this was followed by a lower frequency of stage III (18.9%), stage II (17.5%), and stage IV (9.5%) disease.
Table 2 details the surgical procedures used and the associated outcomes. Distal gastrectomy (DG) was performed in 61.7% of patients, pylorus-preserving gastrectomy (PPG), total gastrectomy (TG), and proximal gastrectomy (PG) were performed in 2.8%, 30.5%, and 4.0%, respectively. Approximately 35% of patients underwent laparoscopic gastrectomy. The gallbladder (19.3%) was frequently resected during gastrectomy. The combined resection rate for the spleen was 6.5%, which was lower than the 9.3% frequency observed in the previous period between 2001 and 2007 [4]. D2 lymphadenectomy was the most common type of lymphadenectomy performed (44.4% of patients). Modified D1+ gastrectomy was performed in 37.2% of patients. Surgery with a sufficiently wide margin was completed in 97.3% of patients. R0 resection was performed in 90% of patients.
The median number of dissected nodes was 33 in all the patients, 34 each in those who underwent laparotomy, thoracotomy, and 32 in those who underwent laparoscopic approach. The median number of dissected nodes was 14 for D0 lymphadenectomy, 23 for D1 lymphadenectomy, 31 for D1+ lymphadenectomy, 38 for D2 lymphadenectomy, and 46 for D2+ lymphadenectomy.
Of the 92,305 patients who underwent gastrectomy, 441 died within 30 days of surgery, yielding a 30-day operative mortality of 0.5%. Furthermore, 905 (1.0%) died within 60 days of surgery, and 1460 (1.6%) died within 90 days of surgery. In total, 25,946 patients died during follow-up. Most often, the cause of death in patients who underwent gastrectomy was the primary disease (n = 15,986), followed by death from other diseases (n = 5089) and other cancers (n = 1933). The most common type of recurrence was peritoneal metastasis (41.3%, 6754/16,353), followed by hematogenous metastasis (30.5%, 4990/16,353), lymph node metastasis (19.3%, 3164/16,353), and local recurrence (7.0%, 1148/16,353) (Table 3a). The predominant mode of recurrence in patients with tumor without serosal invasion (M, SM, MP, SS) was hematogenous metastasis. In patients with tumors that had invaded the serosa (SE, SI), the predominant mode of recurrence was peritoneal metastasis, followed by hematogenous metastasis. Recurrence rates between 2010 and 2013 (Table 3a) were not lower than the rates between 2004 and 2009 (Table 3b).
The 5-year OS rate in the 92,305 patients who underwent resection of the primary gastric cancer was 70.6% (95% confidence interval 70.2–71.0%; Table 1). OS and the disease-specific survival distributions differed between males and females. Significant differences in the prognosis were observed among the disease stages (Table 1, Fig. 3). Subgroup analyses of the survival distribution as classified by the pT and pN category are shown in Table 4. The survival for each stage according to the age group is summarized in Table 5. Elderly patients had poorer survival, in terms of both OS and disease-specific survival; this was true for every stage of the disease.
Table 6 shows the proportions of node-positive patients, the 5-year OS rates, and the 5-year disease-specific survival rates; the table allows comparison of the outcomes of reduced-extent lymphadenectomy, reduced-extent gastrectomy, and standard D2 gastrectomy for early gastric cancer. The D2 gastrectomy group had a higher number of node-positive patients than either the D1 or D1+ group. In all early gastric cancer groups (pT1a and pT1b), the 5-year OS rate of those who underwent D1 gastrectomy was lower than that of those who underwent D1+ or D2 gastrectomy. In contrast, the 5-year disease-specific survival rates were similar. The outcomes of reduced-extent gastrectomy and standard D2 gastrectomy were similar to those of reported for 2001–2007 [4].
Discussion
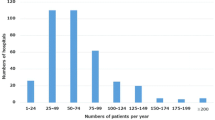
The annual number of gastric cancer cases registered to the nationwide database in Japan has been gradually increasing. The estimated number of gastric cancer incidence cases in Japan in 2013 was 131,893 [12]. The national clinical database (NCD) showed 39,957 cases of distal gastrectomy and 19,035 cases of total gastrectomy in 2013 [13]. The NCD covers more than 95% of operative procedures performed by surgeons in Japan and includes many diseases other than gastric cancer. The data in this study showed 14,315 cases of distal gastrectomy and 6915 of total gastrectomy in 2013, which accounted for approximately 36% and 36% of the cases from NCD, respectively. The median patient volume in the participating hospitals in 2013 was 54 patients (1–511) per year. Therefore, the participating hospitals were rather representative ones including high-volume centers.
As life expectancy increased, the median age of registered patients with gastric cancer has also increased. Patients with cancer located in the upper third of the stomach accounted for 21.4% of cases (2399/11,218) in 2001 and 21.7% (4984/22,977) in 2013. Although esophagus-invading tumors were identified, this registry does not allow the discrimination of junctional tumors. The proportion of cases diagnosed as early gastric cancer was approximately 50%, which has been stable for surgical cases. It is estimated that endoscopic treatments, mainly for mucosal cancer [14], account for more than 60% of early gastric cancer treatments in Japan [15]. This study showed the proportion individuals with surgically treated mucosal cancer gradually decreased from 27% in 2001 to 21% in 2013.
The frequency at which each resection method was used did not change in the 2001–2013 period. Almost all were treated by standard distal or total gastrectomies. Laparoscopic approach increased dramatically from 2001 to 2013. Combined resection was performed in 30% of the patients in 2010–2013, which slightly decreased than the 32% figure from 2001 to 2007 [4]. Especially, combined resection of the spleen decreased from 9.3% in 2001–2007 to 6.5% in 2010–2013. As for extent of lymph node dissection, standard D2 dissection was performed for 47% of patients in 2001–2007 [4] and 44% of those in 2010–2013. Modified gastrectomy, D1 + α and D1 + β gastrectomy was done in 28% of patients in 2001–2007 [2] and D1+ gastrectomy was performed in 37% of those in 2010–2013. From 2001 to 2013, the guidelines, which recommends standard and modified gastrectomies, might be getting used in widespread.
As Table 3a shows, hematogenous recurrence was the major mode of recurrence for tumors without serosal invasion. In contrast, peritoneal recurrence became predominant when the tumor invades the serosa. When compared to the data from 2004 to 2009 (Table 3b), no remarkable change was found on recurrence site and rates. The efficacy of S-1 adjuvant chemotherapy for stage II and III disease was confirmed in 2007 [16]. We checked the time trend of recurrence rate by tumor depth (Suppl. Table), however, there was no obvious improvement.
The 5-year OS rate after resection in 2010–2013 was 70.6% of all patients, which was a slight improvement from 68.2% in 2001–2007 [4]. As the classification was revised between the 2nd version and the 3rd version since 2010, care must be taken when comparing survival rates between patient cohorts whose disease may have been classified differently for historical reason. A comparison of the 5-year disease-specific survivals (DSS) of the patients in this study (2010–2013) to those in 2001–2007 [4] revealed that the 5-year DSS of patients with pT1 (M, SM) tumors was similar, at 98.2% [4] and 98.3% (Table 4), respectively. The M to SM tumor ratio was 0.97:1 in 2001–2007, and 0.79:1 in 2010–2013. The proportion of M tumors treated by endoscopic resection had increased in 2010–2013 when compared to the earlier period. The 5-year DSS of the patients with SS, SE, and SI tumors slightly improved from 69.8%, 39.0%, and 29.2% [4] to 72.6%, 42.2%, and 31,3% (Table 1), respectively.
As for reduced extent of lymph adenectomy, the 5-year DSS rates associated with D1 or D1+ gastrectomy were not inferior to that associated with D2 gastrectomy (Table 6). It could be that D1 gastrectomies were often performed for frail patients, which might explain why the 5-year OS rates of D1 gastrectomy patients were lower than those who underwent D1+ or D2 gastrectomy.
A major limitation of this study relates to its retrospective design, which cannot evaluate enough each procedures including reduced surgery for pT1 tumors on the basis of the results of this study alone. And, the follow-up rate was not so high, which was 84.7% in 2001–2007, however, that improved to 89.5% in 2010–2013.
The registration committee of the JGCA and NCD planned to apply the same registration items of the conventional JGCA gastric registry to the NCD gastric cancer registry. The NCD gastric cancer registry started in 2018 and enrolled the patients who underwent gastrectomy in 2011 [17]. After a 3-year trial period (2011–2013) to compare the reliability of both registries, the NCD gastric cancer registry has continued to enroll patients since 2014. The success of the NCD registry will increase the number of hospitals participating in the cancer registry and accelerate the complete enumeration of the gastric cancer registry.
Conclusion
Our detailed analysis highlights historical changes in the outcomes of surgically treated gastric cancer in Japan from 2001 to 2013, and provides a robust dataset for future analysis.
References
Japanese Research Society for Gastric Cancer, Japanese Gastric Cancer Association. Zenkoku igan touroku chousa houkokusyo (Statistical report of nationwide registry of gastric carcinoma), no. 1–54. Tokyo: National Cancer Center; 1972–2001.
Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14(4):301–16.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21:144–54.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–12.
Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–23.
Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100.
International Union Against Cancer. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. New Jersey: Wiley-Blackwell; 2009.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10–24.
Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 5th ed. New York: Wiley-Liss; 1997.
Cancer Information Service, National Cancer Center, Japan. Cancer Incidence (1975–2015) Table download. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fganjoho.jp%2Freg_stat%2Fstatistics%2Fdata%2Fdl%2Fexcel%2Fcancer_incidence(1975-2015).xls&wdOrigin=BROWSELINK.
Marubashi S, Takahashi A, Kakeji Y, Hasegawa H, Ueno H, Eguchi S, et al. Surgical outcomes in gastroenterological surgery in Japan: report of the National Clinical Database 2011–2019. Ann Gastroenterol Surg. 2021;5(5):639–58.
Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33(1):4–20.
Ono H. The history, present status and future perspective of ESD for GI tract cancer. Nihon Shokakibyo Gakkai Zasshi. 2017;114:971–7 (Japanese).
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Suzuki S, Takahashi A, Ishikawa T, Akazawa K, Katai H, Isobe Y, et al. Surgically treated gastric cancer in Japan: 2011 annual report of the national clinical database gastric cancer registry. Gastric Cancer. 2021;24(3):545–66.
Acknowledgements
The Japanese Gastric Cancer Association Registration Committee appreciates the great effort of the participating hospitals in registering accurate and detailed data for this project.
Funding
The authors declare that this study was not funded.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and in compliance with the Helsinki Declaration of 1964 and latter versions. Every hospital discloses information to the patients about the nationwide registry of the Japanese Gastric Cancer Association. Participating patients were excluded only when they specified that they were unwilling to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kakeji, Y., Ishikawa, T., Suzuki, S. et al. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001–2013). Gastric Cancer 25, 1082–1093 (2022). https://doi.org/10.1007/s10120-022-01317-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01317-6