Abstract
Background
Signet ring cell carcinoma (SRC) is a distinct subtype of gastric cancer (GC); however, the specific characteristics of cancer cell surface glycans and glycosylation remain unclear. In this study, we investigated SRC-specific glycans using lectin microarray and evaluated the potential applicability of a glycan-targeting therapy.
Methods
SRC cell lines (NUGC-4 and KATO-III) and non-SRC (NSRC) cell lines (NCI-N87, SNU-1, and MKN-45) were subjected to lectin microarray analysis to identify the SRC-specific glycans. Additionally, we performed immunohistochemical lectin staining and evaluated the anti-tumor effects of lectin drug conjugates (LDCs) using high-affinity lectins for SRC.
Results
Among the 96 lectins tested, 11 high-affinity and 8 low-affinity lectins were identified for SRC. Glycan-binding motifs varied in the high-affinity lectins, but 5 (62.5%) low-affinity lectins bound the same glycan structure, α2–6-linked sialic acids. The ratio of signal intensity in SRC to NSRC (SRC/NSRC) was highest in the rBC2LCN lectin (1.930-fold), followed by the BPL lectin (1.786-fold). rBC2LCN lectin showed high affinity for both SRC cell lines and one of the three NSRC cell lines (NCI-N87). The therapeutic effects of the LDC, rBC2LCN-PE38 (rBC2LCN, and Pseudomonas exotoxin A), showed cytocidal effects in vitro and tumor regression in in vivo mouse xenograft models.
Conclusion
We reported specific glycan profiles in SRC cells, showing reduced α2–6-linked sialic acids. Additionally, we found a targeted therapy using rBC2LCN lectin might be applicable as an alternative treatment option for patients with SRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Signet ring cell carcinoma (SRC) is a histological subtype of gastric cancer (GC) and is defined as an adenocarcinoma in which more than 50% of the tumor cells are scattered malignant cells containing intracytoplasmic mucin, according to the World Health Organization (WHO) histological classification of tumors [1]. This phenotype is often associated with “diffuse type” growth in the Lauren classification [2], and is characterized by scirrhous type growth with abundant fibrosis [3]. Several studies have reported that gastric SRC has distinct clinicopathological characteristics in comparison with non-SRC (NSRC); however, the prognosis of SRC is still controversial [4]. Despite the development of new chemotherapeutic agents, including molecular targeting drugs, an effective cancer targeting therapy has not been established for SRC. This could be because SRC has few specific genomic mutations and is classified as a genomically stable (GS) subtype in The Cancer Genome Atlas (TCGA) [5].
Glycans cover the outermost layer of the cell membrane. Glycosylation is a common protein modification in humans, and aberrant glycosylation plays a role in tumor invasiveness, metastasis, and prognosis [6]. Several studies have reported that glycosylation in GC and lectins which bind to specific glycosylation structures, are associated with GC and its prognosis [7]. In comparison with normal tissue or gastric ulcer tissues, GalNAc glycans were highly expressed in GC and its binding lectin, Vicia villosa agglutinin (VVA), was associated with lymph node metastases [8]. Decreased expression of BPL lectin, which binds to Galβ1-3GalNAc structure, in GC tissues showed poor prognosis due to cancer recurrence [9].
SRC has distinct clinicopathological characteristics in comparison with other types of gastric carcinomas. However, the cell surface glycan expression and glycosylation in SRC have not been defined till date. Therefore, we investigated the glycan expression profile of SRC by comparing it with that of NSRC using lectin microarray and immunohistochemistry. Additionally, we selected rBC2LCN lectin, which showed the most prominent SRC/NSRC ratio, and evaluated the potential applicability of a novel cancer targeting therapy using a lectin drug conjugate (LDC).
Materials and methods
Cell lines
Human gastric adenocarcinoma cell lines, KATO-III, SNU-1, and NCI-N87 were purchased from ATCC (Manassas, VA, USA); NUGC-4 was purchased from RIKEN BioResource Research Center (Tsukuba, Ibaraki, Japan), and MKN-45 was purchased from the Japanese Collection of Research Bioresources cell bank (Sennan, Osaka, Japan). KATO-III and NUGC-4 are signet ring cell GC cell lines, which we defined as SRC cell lines. NCI-N87 is a well differentiated, and SNU-1 and MKN-45 are poorly differentiated gastric adenocarcinomas without signet ring cells, which we defined as NSRC. The attributes of the aforementioned cell lines were confirmed using the Cancer Cell Line Encyclopedia. SNU-1 and NCI-N87 cells were cultured in RPMI 1640 (ATCC), KATO-III cells in IMDM (ATCC), and MKN-45 and NUGC-4 cells in RPMI 1640 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The media were supplemented with 10% or 20% FBS (GIBCO, Paisley, RF, UK) and 1% penicillin–streptomycin (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). All cells were incubated at 37 °C in a 5% CO2 atmosphere, and were passaged directly or via trypsinization.
Patient samples and tissue collection
Thirty-two pairs of formalin-fixed and paraffin-embedded (FFPE) human GC tissue sections and frozen adjacent normal tissues were obtained from the Department of Pathology, University of Tsukuba Hospital, Tsukuba, Japan. All clinical samples were obtained with written informed consent from the patients. All the protocols followed the regulations relating to ethics of human subject research at the University of Tsukuba, Tsukuba, Japan. This study was approved by the Tsukuba Clinical Research and Development Organization (T-CReDO), Tsukuba, Japan.
Protein extraction
A total of 5 × 104–107 live cells, or frozen tissue sections were lysed with CelLytic MEM Protein extraction kit (Sigma-Aldrich, St. Louis, Missouri, USA) and extracted membrane protein fractions. The protein concentrations were determined using the Micro BCA™ Protein Assay kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA), and γ-globulin was used as the standard (Bio-Rad Laboratories, Hercules, California, USA). The procedures were performed according to the manufacturer’s instructions.
Lectin microarray
A lectin microarray was constructed and analyzed as previously described [10]. In brief, 96 lectins were immobilized, and the protein samples were labeled with the fluorescent dye Cy3 (GE Healthcare, Boston, Massachusetts, USA). The Cy3-labeled lysates were applied to each well of the microarray slides and incubated at 20 °C overnight, and the chips were scanned using an evanescent field-activated fluorescence scanner (Bio-REX Scan 200, Rexxam Co., Ltd, Osaka, Japan). Automatic intensity analysis was performed after high-speed scanning, and the quantified signal was analyzed using Cluster 3.0 (yellow: high; blue: low; black: intermediate), mean-normalized, log-transformed, and analyzed via the average linkage method.
Lectin staining
Immunohistochemistry FFPE tissue blocks were prepared as 3 μm sections, and autoclaved for antigen retrieval. Endogenous hydrogen peroxide activity was blocked with 3% hydrogen peroxide in methanol. The lectin, rBC2LCN, was labeled with horseradish peroxidase and applied to the blocked sections, and 3,3′-diaminobenzidine (Histofine SAB-PO(M) kit, Nichirei Bioscience, Tokyo, Japan) was added. The stained slides were scored according to average staining intensity and positivity in cancer cell membranes, which were categorized as highly positive (3 +), positive (2 +), slightly positive (1 +), and negative (0).
Live cell staining Live cells (3 × 105 cells) were seeded in 35 mm glass dishes. After 48 h of seeding, the dishes were washed with PBS three times. The cells were incubated at 4 °C for 30 min to inhibit endocytosis, and then 1 μL of rBC2LCN-FITC (AiLecS1-FITC) (Wako Pure Chemical Corporation, Osaka, Japan) in 1 mL of medium. The cells were incubated at 37 °C in a CO2 incubator or 4 °C for 1 h, and then the medium was replaced with PBS. Images were captured using a BIOREVO BZX-710 fluorescence microscope (Keyence, Osaka, Japan).
Flow cytometry
Lectins were labeled with phycoerythrin (PE) and adjusted to 1 mg/mL. Cells were harvested and washed twice with 1% BSA/PBS, and 1 μL of the pretreated lectins was added to each tube. After one hour incubation and washing, we obtained cells that were bound to the lectin rBC2LCN. The cells were analyzed using Cell Sorter SH800s (SONY, Tokyo, Japan), and fluorescence signals were determined using FlowJo (v10.5, BD Biosciences, New Jersey, USA).
Cell viability assay
rBC2LCN-PE38, a conjugate of rBC2LCN lectin with a 38 kDa domain of Pseudomonas exotoxin A containing the domains Ib and II in addition to domain III (PE38) [11] was used as a LDC treatment experiments. Commercially available rBC2LCN-PE38 conjugate, stem secure human pluripotent stem cell (hPSC) remover was purchased (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The viability of cells treated with rBC2LCN-PE38 was determined using the CCK-8 kit (Dojindo Molecular Technologies, Kumamoto, Japan). The cells were seeded at 5 × 103 cells/100 μL/well in 96-well plates and cultured for 48 h. On the third day after seeding, the rBC2LCN-PE38 conjugate was added, diluted to multiple concentrations ranging from 1 × 106 to 0 pg/mL and incubated for 48 h at 37 °C in a CO2 incubator. On day 5, 10 μL of CCK-8 was added per well, and after 2 h of incubation, the absorbance was measured at 450 nm using a SpectraMax M3 spectrophotometer (Molecular Devices LLC, San Jose, California, USA). Data were analyzed using GraphPad Prism 9.0.
Animal models
Subcutaneous cell xenograft model: A total of 2 × 106 NCI-N87 and NUGC-4 cells were suspended in 200 μL PBS and subcutaneously injected into nude mice (BALB/cA-nu, female, 5 weeks old, CLEA, Japan). Animal experiments were performed in accordance with the ethical guidelines set by the Animal Care Committee of the University of Tsukuba, Tsukuba, Japan.
LDC treatment for in vivo GC mouse models
Tumors were allowed to grow close to 100 mm3, or for 14 days before LDC treatment. After the nodules were established, nodules from one mouse in each group were excised and were prepared as FFPE sections to examine the morphology and perform lectin staining to confirm the affinity to rBC2LCN, and the remaining mice were randomly allocated to two groups (5 mice per group). The rBC2LCN-PE38 was diluted into 1 μg/300 μL/mouse in PBS and administered by intraperitoneal injection on days 1, 3, 5, 8, 10, and 12, and PBS was administered to the control group in the same way. A digital caliper was used to measure the width and length of the tumor on the day of injection. Tumor volume was determined using the following formula: 0.5 × (width)2 × (length). On day 15, the mice were sacrificed and the tumor nodules were excised, followed by weight measurement.
Statistical analysis
Cluster 3.0 and Java tree view were used to perform cluster analysis, and the results were exported as heat maps. Student’s t test in SPSS Statistics 21.0 (SPSS Inc.) was used to evaluate the differences in the continuous variables. Categorical data comparisons between multiple groups were performed by analysis of chi-square test followed by Bonferroni’s posttest analysis. Differences were considered statistically significant at p < 0.05. The cell viability curve satisfied goodness-of-fit. All other analyses were performed using the GraphPad Prism 9.0.
Results
Lectin microarray analysis of GC cell lines
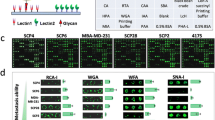
The glycan expression profile in SRC cell lines was different from that of the NSRC cell lines (Fig. 1A). A total of 19 lectins showed significantly different binding affinities between SRC and NSRC. Eleven lectins (BPL, rDiscoidin II, rPALa, GSL-I A4, SBA, ECA, rSRL, DSA, rBC2LCN, CCA, and rBC2LA) showed higher affinity for SRC cell lines, while eight lectins (TJAI, SNA, SSA, rACG, rPSL1a, PVL, rLSLN, and STL) showed lower affinity (Table 1). BPL lectin showed most significant signal intensity difference between SRC and NSRC (p < 0.001). None of the 11 high-affinity lectins for SRC showed common specific glycan binding structures or glycosylation. In contrast, five (62.5%) of the eight low-affinity lectins had the same glycan binding structure, α2–6-linked sialic acids (α2–6 Sia). The ratio of SRC signal intensity to NSRC was the highest in rBC2LCN lectin (1.930-fold), followed by BPL lectin (1.786-fold) (Fig. 1B, C). The signal intensity of rBC2LCN lectin in both SRC cell lines was high; KATO-III (75.4 ± 28.5) and NUGC-4 (57.7 ± 15.8) (Fig. 1D). In the NSRC cell lines, two cell lines had low signal intensity, SNU-1 (25.5 ± 7.9) and MKN-45 (24.0 ± 6.2), whereas NCI-N87 (53.9 ± 7.7) showed a high signal intensity, similar to the that of the SRC cell lines.
Lectin microarray analysis of gastric cancer (GC) cell lines. A A total of 96 lectins were analyzed, and specific lectins showed different affinities to the glycan on the surface of SRC or NSRC cells (yellow: high; black: intermediate; blue: low). B A total of 19 lectins were significantly different (p < 0.05) between the two groups; 11 lectins (orange) showed higher binding affinity, and eight lectins (blue) had lower affinity for SRC cells. C) Signal intensity ratio of SRC to NSRC (SRC/NSRC). D) Signal intensity of rBC2LCN in all five GC cell lines. *p < 0.05; **p < 0.01
Lectin selection for SRC-targeted therapy
BPL showed most significant signal intensity difference between SRC and NSRC, we therefore considered it as a candidate for SRC-targeted lectin at first. However, BPL lectin showed hemagglutination except for O-type blood and it was found not to be applicable in vivo (Fig. S1). We then next selected rBC2LCN which showed highest SRC/NSRC ratio, and it already established in vivo applicability in our previous experiments for pancreatic ductal adenocarcinoma (PDAC) [12]and colorectal cancer (CRC) [13].
Lectin affinity assay for rBC2LCN
We confirmed the affinity between rBC2LCN and GC cell lines (both SRC and NSRC cell lines) by live-cell staining (Fig. 2A). KATO-III, NUGC-4, NCI-N87 and MKN-45 were stained for cancer cell membranes by rBC2LCN, while SNU-1 was not. After binding to cell surface glycans, rBC2LCN was internalized to cytoplasm by endocytosis (Fig. S2). We also confirmed the affinity of rBC2LCN by histochemical staining of cell line-derived xenograft models, except for KATO-III, which could not establish xenograft tumors (Fig. 2B, C). NUGC-4 and NCI-N87 were highly positive for rBC2LCN lectins, whereas MKN-45 was weakly positive, and SNU-1 was negative. We also confirmed cell surface binding of rBC2LCN with flow cytometry (Fig. 2D). Four cell lines (NUGC-4, KATO-III, NCI-N87, and MKN-45) were positive for rBC2LCN surface binding, but SNU-1 was negative. Among four positive cell lines, robust standard deviation (SD) which means 68.26% of the events around the Median, showed higher in SRC cell lines (NUGC-4: 141,825, KATO-III: 156,641) than NSRC cell lines (NCI-N87: 36,394, MKN-45: 12,340).
Lectin affinity confirmation for rBC2LCN. A Microscopic images of live cell staining of rBC2LCN-FITC (1 µg/mL), B HE staining of cell derived subcutaneous xenografts, C rBC2LCN lectin staining of cell derived xenografts, D Flow cytometry analysis of live cells, blue represents cells labeled with rBC2LCN, red represents cells labeled with phycoerythrin (PE) − rBC2LCN (1 µg/mL each); two experiments have been overlaid, the number shows robust standard deviation (SD), E Cytotoxicity of rBC2LCN-PE38 conjugate in vitro evaluated using CCK-8 assay kit. Scale bar 50 µm
Histochemical lectin staining of rBC2LCN in clinical GC samples
We also evaluated the affinity of rBC2LCN lectin in clinical GC specimens and adjacent normal stomach tissues. Of the 32 clinical cases, all SRC cases (n = 6) showed highly positive staining (greater than 2 +) for rBC2LCN (Fig. 3A, D). In addition, 15 (71.4%) of the 21 poorly differentiated, 8 (88.9%) of 9 moderately differentiated, and 4 (57.1%) of 7 well differentiated adenocarcinomas were strongly positive for rBC2LCN (Fig. 3B, D). Only five (15.6%) of the 32 clinical samples had a focal negative staining area, and none of the samples stained completely negative. In the normal stomach tissues, 6 (18.8%) were negative, 18 (56.3%) were slightly positive (1 +), 8 (25%) were positive (2 +), and none were highly positive (3 +). Positive ratio of greater than 2 + was 8 (25%) in normal tissues, and the ratio was lower than SRC (p = 0.201) and NSRC (p = 0.071); however, those were not significant. Also, the difference between SRC and NSRC was not significant (p = 0.990) (Fig. 3D).
Cytotoxicity of rBC2LCN-PE38 conjugate in vitro
The cytocidal effect of rBC2LCN-PE38 was the highest in KATO-III (IC50 = 46.2 pg/mL), followed by NUGC-4 (IC50 = 89.7 pg/mL) (Fig. 2E). In contrast, NSRC cell lines had higher IC50 values, 1411 pg/mL for MKN-45 and 489.9 ng/mL for SNU-1. However, the IC50 value for NCI-N87 (104.0 pg/mL) was low, similar to that of the SRC cell lines.
Therapeutic efficacy of rBC2LCN-PE38 in vivo
The effect of in vivo treatment of rBC2LCN-PE38 was evaluated using cell line-derived xenograft models, NUGC-4 (SRC) and NCI-N87 (NSRC), as both showed high affinity to rBC2LCN lectin. NUGC-4 derived xenografts showed significantly smaller tumor volume on Day 8 (p = 0.004), 10 (p = 0.04), 12 (p < 0.001), and 15 (p = 0.003) in the LDC group than that in the control group (Fig. 4A). Similarly, NCI-N87 derived xenografts also showed significantly smaller tumor volume on day 3 (p = 0.001), 5 (p = 0.001), 8 (p = 0.003), 10 (p = 0.001), 12 (p = 0.001), and 15 (p = 0.001) in the LDC group than that in the control group (Fig. 4B). However, no difference in LDC drug toxicity was observed between the LDC and control groups in terms of body weight transition (Fig. 4C, D).
Evaluation of the therapeutic efficacy of LDC(rBC2LCN-PE38) in cell line-derived mouse xenograft models. LDC was injected into peritoneum (i.p.) totally six time every 2 or 3 days (arrow). Change of relative tumor volume in A NUGC-4, B NCI-N87, and change of mice body weight during experimental period in (C) NUGC-4, D) NCI-N87). *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The present study reported the characteristics of glycan expression profiles in SRC cells and identified 11 high-affinity and eight low-affinity lectins. Glycan structures which high-affinity lectins bind to varied, but a common glycan structure, α2–6 Sia, was identified among the low-affinity lectins. Therefore, relative downregulation of α2–6 Sia might be related to the specific properties of SRC cells. Among the high-affinity lectins, rBC2LCN lectin showed the highest SRC/NSRC affinity ratio and has potential applicability as an LDC targeted therapy for SRC.
The typical difference in glycan expression profiles between SRC and NSRC cells was the relative downregulation of α2-6 Sia in SRC, which could be detected by TJAI, SNA, SSA, rACG, and rPSL1a lectins. Sialic acids are acidic sugars that are typically located at the outer ends of cell surface glycan chains [14], and an increase in α2-6 sialylation is frequently observed in tumor cells [15]. Expression of α2-6 Sia is mediated by β-galactoside α2-6-sialytransferase (ST6Gal-I), and its increase is correlated with cancer progression, metastatic spread, and poor prognosis [16]. SRC has been historically recognized as a GC subtype with worse prognosis when compared with other GC subtypes, because of its characteristic “diffuse-type” growth and frequent peritoneal metastases in advanced stages [17]. However, recent studies have shown that the prognosis of early-stage SRC is similar to [18, 19] or even better [20, 21] than that of the common type of adenocarcinoma, while advanced-stage SRC is associated with poor prognosis [20, 22]. These results suggest that the signet-ring cells are not intrinsically malignant, but gain malignant properties following submucosal invasion through cell–cell interactions with stromal cells, such as cancer-associated fibroblasts [22]. Therefore, it may be consistent that α2-6 sialylation, which relates malignant properties, is not necessarily high in SRC. In addition, α2-6 sialylation of N-glycans is known as an important modulator of β1 integrin, which affects tumor metastasis by modulating cancer cell adhesion and migration [23,24,25]. Increased α2-6 sialylation of β1 integrin is frequently observed in colon adenocarcinoma through increased activity of ST6Gal-I, which augments tumor metastases [23]. These findings seem to correlate with a higher frequency of hematogenous metastases in colon cancer, and may provide an explanation for the low frequency of hematogenous metastases in SRC [18]. Although further investigation is required, this characteristic finding of low α2-6 Sia may also play a role in cell–cell interactions of poorly cohesive SRC by modulating cell adhesion molecules.
Among the 11 high-affinity lectins, rBC2LCN showed the highest SRC/NSRC ratio, followed by BPL. The binding structures of glycans for these two lectins are not the same, but share a common motif, Galβ1-3GlcNAc. This motif is a type 1 glycan chain and is classified as a mucin-type-O-glycan [26]. Mucinous proteins are decorated by hundreds of O-linked glycans and glycosylation patterns, making them important in many cancer-associated biological processes [27]. Therefore, our results showing high affinity for these O-glycans in SRC are understandable. Moreover, immunohistochemical staining of rBC2LCN lectins using clinical GC samples showed strongly positive (score 2 or more) staining in all SRC samples, but the staining pattern was not specific for SRC. An attempt to classify SRC based on mucin expression level has been reported: gastric type (MUC1, MUC5AC, and MUC6), intestinal type (MUC2 and CDX2), and mixed gastrointestinal phenotypes [28]. However, a recent review concluded that there is no immunohistochemical mucin stain unique to SRC cancer nor are there differences with other GC subtypes [29]; thus, our results where most NSRC samples stained positive, were not surprising. In addition, discrepancies in glycan expression among human GC tissue samples and GC cell lines were reported previously [8]. Yamashita, et al. reported that VVA lectin which was not expressed in seven GC cell lines was expressed in clinical tissue samples. VVA lectin has a binding affinity for the Tn antigen, which is also a mucin-type glycan. Our results were consistent with their report; all GC cell lines used in this study showed low VVA lectin expression. They suggested that the discrepancy was caused by carcinoma–stroma interactions, and this hypothesis seems acceptable because glycans and glycosylation are dynamically modulated by cell–cell interactions [16].
We have previously shown that the rBC2LCN lectin has specificity for PDAC [12] and CRC [13] cell surface glycans (Fuc α1-2Galβ1-3GlcNAc(GalNAc)- epitopes, such as H type 1/3/4). This finding was validated for SRC in the present study. Increased levels of fucose and fucosylation, resulting in enhanced expression of fucosyltransferase (FUT), are frequently observed in cancer patients [30]. The elevated expression of FUT4 in GC is correlated with tumor progression, and carcinogenesis of GC induced by CagA, following Helicobacter pylori infection [31]. rBC2LCN lectin was originally identified as a probe specific for human embryonic stem cells and human induced pluripotent stem cells[30,31,32], and rBC2LCN-positive cancer cells are associated with cancer stem-like characteristics in prostate cancer [33]. Therefore, higher expression of rBC2LCN in SRC, a poorly differentiated GC subtype, is an understandable finding. We have identified five membrane glycoproteins that are highly expressed in PDAC but minimally expressed in normal pancreatic tissues: carcinoembryonic antigen 5 (CEA), integrin beta 1 (ITGB1), mucin 16 (MUC16), prostate stem cell antigen (PSCA), and glucose transporter type 1 (GLUT1) [34]. Among them, CEA showed the highest specificity; therefore, the result of this study that rBC2LCN showed high specific affinity for GC in which is frequently expressed CEA in tumor tissues [35] is considered to be acceptable.
rBC2LCN-PE38 is a conjugate of rBC2LCN lectin with the Pseudomonas exotoxin (PE)-A that we previously developed [12]. This LDC showed cytotoxic activity against rBC2LCN-positive PDAC and CRC without remarkable toxicity, including hemagglutination[12, 13]. The efficacy of rBC2LCN-PE38 against SRC and rBC2LCN-positive NSRC was reproduced in this study. These results shed new light on GC treatment, including that of SRC. Molecular targeting agents have improved survival outcomes in various cancers; however, many phase III trials have failed to demonstrate a survival benefit in GC. SRC is classified as a GS subtype in TCGA [5] and has few specific mutations limiting biomarkers for targeted therapy approaches. Historically, lectins have been recognized as toxins that cause hemagglutination in animals; rBC2LCN lectin, on the contrary, has unique properties and lacks this typical drawback. We are now developing a novel LDC drug targeting rBC2LCN for pancreatic cancer in the preclinical trials; thus, it might be a novel treatment option for patients with SRC in future.
This study has several limitations. First, we classified and compared SRC and NSRC based on the origin of the cell lines, but the clinical definition of SRC is controversial. The histological definition of SRC is inconsistent; the WHO definition of SRC has changed several times between the 1st edition (1977) and the 4th edition (2010) [29]. Therefore, these results may not be applicable to clinical patients with SRC. Second, the biological mechanisms of low α2-6 Sia expression in SRC cells were not explored in this study. Therefore, further investigation on this front is required. Third, the anti-tumor effect of the LDC was observed only in mouse ectopic xenograft models; thus, clinical applicability should be further evaluated. Also, we did not explore the in vivo experiments using xenograft models using low-affinity cell lines for rBC2LCN; however, we previously evaluated the in vivo difference among high and low-affinity cell lines in PDAC [11] and CRC [12], then the different tumor regression among those models was expected. Additionally, we have noted that rBC2LCN lectin binding was not highly specific for cancer cells alone, but normal adjacent stomach tissues. Though staining intensity of immunohistochemistry is relatively lower in normal stomach tissues (Fig. S3A-D) than cancer cells, goblet cells in small (Fig. S3E) and large intestine (Fig. S3F) showed higher positivity for rBC2LCN; therefore, we should carefully evaluate the LDC safety in future. In our previous experiments [11], stomach mucosa showed stronger positivity of rBC2LCN than human, but peritoneum was negative in murine models. Peritoneal dissemination is frequently observed in SRC; therefore, intraperitoneal administration of rBC2LCN-PE38 might be more feasible than intravenous systemic administration in the step of clinical application.
In conclusion, we reported that specific glycan expression in SRC cells and lower expression levels of α2-6 Sia might be key features for understanding the biology of SRC cells. In addition, SRC showed higher affinity for rBC2LCN lectin; therefore, LDC using this lectin might be an alternative treatment option for genomically stable SRC.
References
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–8.
Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Endo K, Sakurai M, Kusumoto E, Uehara H, Yamaguchi S, Tsutsumi N, et al. Biological significance of localized Type IV scirrhous gastric cancer. Oncol Lett. 2012;3:94–9.
Zhang C, Liu R, Zhang WH, Chen XZ, Liu K, Yang K, et al. Difference between signet ring cell gastric cancers and non-signet ring cell gastric cancers: A systematic review and meta-analysis. Front Oncol. 2021;11:1–12.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
You X, Wang Y, Wu J, Liu Q, Chen D, Tang D, et al. Galectin-1 promotes metastasis in gastric cancer through a sphingosine-1-phosphate receptor 1-dependent mechanism. Cell Physiol Biochem. 2018;51:11–30.
Xiang L, Feng G, Dongliang L, Zengqi T, Ganglong Y, Yanli W, et al. Identification of aberrantly expressed glycans in gastric cancer by integrated lectin microarray and mass spectrometric analyses. Oncotarget. 2016;7:87274–300.
Yamashita K, Kuno A, Matsuda A, Ikehata Y, Katada N, Hirabayashi J, et al. Lectin microarray technology identifies specific lectins related to lymph node metastasis of advanced gastric cancer. Gastric Cancer. 2016;19:531–42.
Futsukaichi T, Etoh T, Nakajima K, Daa T, Shiroshita H, Shiraishi N, et al. Decreased expression of Bauhinia purpurea lectin is a predictor of gastric cancer recurrence. Surg Today. 2015;45:1299–306.
Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, et al. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat Methods. 2005;2:851–6.
Tateno H, Saito S. Engineering of a Potent Recombinant Lectin-Toxin Fusion Protein to Eliminate Human Pluripotent Stem Cells. Molecules. 2017;22.
Shimomura O, Oda T, Tateno H, Ozawa Y, Kimura S, Sakashita S, et al. A novel therapeutic strategy for pancreatic cancer: Targeting cell surface glycan using rBC2LC-N lectin-drug conjugate (LDC). Mol Cancer Ther. 2018;17:183–95.
Kitaguchi D, Oda T, Enomoto T, Ohara Y, Owada Y, Akashi Y, et al. Lectin drug conjugate therapy for colorectal cancer. Cancer Sci. 2020;111:4548–57.
Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–9.
Hedlund M, Ng E, Varki A, Varki NM. α2-6-linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. 2008;68:388–94.
Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–18.
Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–7.
Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43–53.
Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg. 2004;188:327–32.
Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: Results from a Chinese mono-institutional study. J Surg Oncol. 2011;103:700–3.
Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678–85.
Miki Y, Yashiro M, Moyano-Galceran L, Sugimoto A, Ohira M, Lehti K. Crosstalk between cancer associated fibroblasts and cancer cells in scirrhous type gastric cancer. Front Oncol. 2020;10:1–13.
Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–52.
Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008;314:2941–50.
Christie DR, Shaikh FM, Lucas JA, Lucas JA 3rd, Bellis SL. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J Ovarian Res. 2008;1:3.
Gupta R, Leon F, Rauth S, Batra SK, Ponnusamy MP. A systematic review on the implications of O-linked glycan branching and truncating enzymes on cancer progression and metastasis. Cells. 2020;9:446.
Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–20.
Xiong ZF, Shi J, Fu ZH, Wan HP, Tu LX. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with K-ras mutation. Genet Mol Res. 2017. https://doi.org/10.4238/gmr16029181.
Kerckhoffs KGP, Liu DHW, Saragoni L, van der Post RS, Langer R, Bencivenga M, et al. Mucin expression in gastric- and gastro-oesophageal signet-ring cell cancer: Results from a comprehensive literature review and a large cohort study of Caucasian and Asian gastric cancer. Gastric Cancer. 2020;23:765–79.
Hirakawa M, Takimoto R, Tamura F, Yoshida M, Ono M, Murase K, et al. Fucosylated TGF-beta receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br J Cancer. 2014;110:156–63.
Aziz F, Khan I, Shukla S, Dey DK, Yan Q, Chakraborty A, et al. Partners in crime The Lewis Y antigen and fucosyltransferase IV in Helicobacter pylori-induced gastric cancer. Pharmacol Ther. 2021;232:107994.
Tateno H, Toyota M, Saito S, Onuma Y, Ito Y, Hiemori K, et al. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286:20345–53.
Mawaribuchi S, Haramoto Y, Tateno H, Onuma Y, Aiki Y, Ito Y. rBC2LCN lectin as a potential probe of early-stage HER2-positive breast carcinoma. FEBS Open Bio. 2020;10:1056–64.
Furuta T, Oda T, Kiyoi K, Yusuke O, Kimura S, Kurimori K, et al. Carcinoembryonic antigen as a specific glycoprotein ligand of rBC2LCN lectin on pancreatic ductal adenocarcinoma cells. Cancer Sci. 2021;112:3722–31.
Wang W, Seeruttun SR, Fang C, Chen J, Li Y, Liu Z, et al. Prognostic Significance of Carcinoembryonic Antigen Staining in Cancer Tissues of Gastric Cancer Patients. Ann Surg Oncol. 2016;23:1244–51.
Acknowledgements
We thank Dr. Shuuji Mawaribuchi and the all-laboratory members Cellular and Molecular Biotechnology Research Institute in AIST for all the technical support. We also acknowledge Dr. Ryota Matsuoka for the pathological consultation and the member of Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, University of Tsukuba for their scientific advice. This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI, JP18K16340, 20K09097). We also would like to thank Editage for English language editing.
Funding
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI, JP18K16340, 20K09097).
Author information
Authors and Affiliations
Contributions
YY, YA, TO, OS, and HT conceptualized the study. YY, OS, HT, and TO developed the methodology. YY, SS, KH, YM, TF, DK, YK, and LP performed the experiments. YY, YA, and OS analyzed the data. YY and YA wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest associated with this manuscript.
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later versions. Informed consent to be included in the study, or equivalent, was obtained from all patients. All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2022_1312_MOESM1_ESM.tiff
Supplementary file1 (TIFF 26370 KB) Figure S1.Hemagglutination assay of BPL. The BPL induces erythrocyte aggregation from a human volunteer of blood type-A, type-B, and type-AB. Only blood type-O did not show hemagglutination even in high concentrations (50 μg/mL). A positive control with concanavalin A (Con A) lectin demonstrated aggregation at 3.13 μg/mL. Each test contained n=3 technical replicates
10120_2022_1312_MOESM2_ESM.tiff
Supplementary file2 (TIFF 26370 KB) Figure S2. rBC2LCN-FITC binds to surface membrane of cancer cells in the condition of endocytosis inhibition (4°C, left column), and is internalized by endocytosis at 37°C temperature (right column)
10120_2022_1312_MOESM3_ESM.tiff
Supplementary file3 (TIFF 18705 KB) Figure S3. Lectin staining of rBC2LCN in clinical human normal tissue samples. Left panels show low magnification hematoxylin-eosin (HE) staining and right panels show high magnification lectin staining. A) gastric fundic mucosa, B) gastric pyloric mucosa, C) spasmolytic polypeptide-expressing metaplasia (SPEM), D) intestinal metaplasia, E) small intestine, F) large intestine. Scale bar 50 µm
Rights and permissions
About this article
Cite this article
Yang, Y., Akashi, Y., Shimomura, O. et al. Glycan expression profile of signet ring cell gastric cancer cells and potential applicability of rBC2LCN-targeted lectin drug conjugate therapy. Gastric Cancer 25, 896–905 (2022). https://doi.org/10.1007/s10120-022-01312-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01312-x