Abstract
IL-6 family cytokine leukaemia inhibitory factor (LIF) study has deciphered a variety of effects, in physiology as well as pathology. Despite the sudden arousal in LIF interest in cancers, its study in the gastric cancer (GC) context has been put aside. Only few related studies can be found in literature, most of them investigating IL-6/STAT3 signalling in GC, and not the particular LIF/LIFRβ signalisation. LIF/LIFR has opposing effects depending on the signalling pathways involved. This review relates the pro- and anti-tumorigenic aspects of LIF/LIFR in GC, taking also into account facts from other types of cancer. A better understanding of these issues would undoubtedly help postulate interesting hypotheses and perspectives for future LIF/LIFR study and its use in GC therapies, where options tend to be limited in number and efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
LIF: generalities
Leukaemia inhibitory factor (LIF) is a multifaceted IL-6 family cytokine. This monomeric protein, often modified by glycosylation, binds to its specific LIF receptor (LIFRβ) GP190, allowing the recruitment of the GP130 subunit of the receptor, heterodimerization and induction of several cell signalling pathways involved in many physiological processes [1]. Indeed, LIF was simultaneously identified by several research teams and was attributed different functions as well as different names. Gearing et al. showed that it was able to induce differentiation and inhibit the proliferation of murine myeloid leukemic M1 cells, naming it LIF [2]. Smith et al. called it DIA for Differentiation Inhibiting Activity for its role in the pluripotency maintenance of murine embryonic stem (ES) cells in vitro, still exploited today in ES cell culture [3]. Finally, Moreau et al. named it HILDA, Human Interleukin for DA cells, and demonstrated its pro-proliferation effects in the murine interleukin-3-dependent leukemic cell line DA-la [4]. Today, LIF is also known for its essential role in embryo implantation [5]. Defective embryo implantation, leading to infertility is observed in female under-expressing LIF. In addition, LIF−/− female mice infertility can be counteracted by recombinant LIF single dose injection [5]. LIF is also important for neurogenesis and tissue regeneration after brain or spinal cord injury [6] as well as for muscle stimulation and regeneration [7].
LIF/LIFR signalling
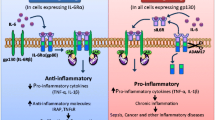
LIF signalisation (Fig. 1) is mediated by LIFRβ-GP130 subunit heterodimerization, preferentially formed when cells are in contact with LIF [8]. GP130 subunit of the receptor is shared with other members of the IL-6 family of cytokines while LIFRβ is shared only with oncostatin M (OSM), cardiotrophin-1 (CT-1), ciliary neurotrophic growth factor (CNTF) and cardiotrophin-like cytokine (CLC). Like most members of IL-6 family, CT-1, CNTF and CLC require an additional LIFRα chain for mediating their signalisation while LIFRβ-GP130 is sufficient for OSM and LIF signalisation [8]. LIF/LIFR canonical pathway is the JAK-induced STAT3 phosphorylation after LIF/LIFR interaction, STAT3 homodimerization and nuclear translocation followed by the induction of target genes for example c-MYC, c-FOS, MMP2 mostly involved in pro-oncogenic processes through their role in survival, differentiation, invasion and in apoptosis inhibition [9,10,11]. The proximal parts of IL-6 receptors are constitutively bound to JAKS [8, 12], but LIF fixation on its receptor can trigger many other signalling cascades. At least three of the four JAK family members (JAK1, JAK2 and TYK2) can bind to LIFR, but JAK1 seems to be the dominant one since LIF/LIFR signalling is highly affected in its absence [13]. JAK1 phosphorylates different tyrosine residues on GP130 and GP190, upon LIF fixation, which serves as scaffold for proteins allowing different signalisation processes. For instance, phosphorylation of Y767, Y814, Y905 and Y915 in GP130 and Y981, Y1001, Y1028 in GP190 allows STAT3 fixation while that of Y759 in GP130 and Y974 in GP190 allows GRB2 recruitment via its SHP2 domain for MAPK pathway [8] (Fig. 1). LIF/LIFR induces MAPK/ERK pathway through GRB2 phosphorylation leading to RAF and RAS activation followed by MEK and ERK activating phosphorylation [14]. LIF/LIFR main feedback mechanisms involve SOCS3 protein whose expression is induced by STAT3. SOCS3 can attach to JAK1 and GP130 to promote their ubiquitination or inhibit JAK activity [15, 16]. Interestingly, SOCS3 fixation sites on GP130 and GP190 are the same as those of GRB2 since SOCS3 also binds via its SHP2 domain [15] (Fig. 1). SOCS3 thus also inhibits LIF-induced MAPK signalling by binding competition. LIF/LIFRβ can also trigger AKT/mTOR signalisation pathway [17] and Ali et al. demonstrate the implication of many other signalling pathways including Rap1, PI3K-AKT and Hippo signalling downstream LIF/LIFR and suggests different effects of LIF/LIFR depending on the different pathways [18].
LIF is being more and more studied in pathological context and more particularly in the cancer context where pleiotropic contradictory effects are being demonstrated. LIF/LIFR signalling is found to be anti-metastatic in breast cancer [19], hepatocellular carcinoma (HCC) [20] and clear cell renal carcinoma [21] while it stimulates melanoma cell migration [22], oesophageal adenocarcinoma treatment resistance [23] and chordoma aggressiveness [24]. In pancreatic cancer, both anti-metastatic [25] and pro-tumorigenic [26] effects of LIF were described.
LIF/LIFR in gastric cancer
Pro-tumorigenic aspects
Despite the knowledge acquired in many cancers, LIF/LIFR in gastric cancer (GC) is still not thoroughly understood. Terawaki et al. have associated cancer cachexia (CC) syndrome, where cancer subjects become frail and lose weight, with an increase in LIF plasma levels in a rat model of GC-associated CC [27] (Fig. 2). The authors demonstrate that surgical ablation of the rat tumours decrease CC symptoms as well as LIF plasma level and suggest Toll-like receptor (TLR) 5 signalling to be involved in LIF-induced CC mechanism. Another study showed that TLR2 upregulation promotes gastric carcinogenesis and is associated to GC patient low survival, thus providing another link between TLRs, GC and IL-6 family signalling [28] (Fig. 2). Here, the authors identify STAT3 as being involved in TLR2 overexpression [28].
In GC, STAT3 was shown to be pro-tumorigenic through the regulation of tumour growth and vascularization in mice models lacking STAT3 negative feedback regulators SHP2/SOCS3 binding sites [29]. Interestingly, these mouse models mimic one of the gastric tumours-promoting capacity of carcinogen type I bacterium Helicobacter pylori, which is the disruption of intracellular SHP2 signalling by bacterium oncogenic protein CagA. SHP2 disruption leads to less feedback regulation of STAT3 and an increase in its activity [29, 30] (Fig. 2). In this regard, the authors found STAT3 hyper-activation in H. pylori-infected antrum and especially in intestinal histological subtype of GC compared to non-tumorous gastric tissue. Fukui et al. found more inactivated methylated SOCS3 in non-neoplastic mucosa of patients with early GC that those without GC [31]. They also demonstrated a correlation between STAT3 activating phosphorylation, SOCS3 methylation and the proliferation marker Ki67. SOCS3 methylation seems to be linked to early GC cells proliferation. Furthermore, Zhang et al. show STAT3 over-activation in late GC as well as in GC lymph node metastasis cases [32]. STAT3-SOCS3 dysregulation can also be attributed to SOCS3 regulation by miRNAs. miR-340, seen to be overexpressed in GC, can target 3’-UTR regions of SOCS3 mRNA and lead to SOCS3 under-expression in GC compared to non-tumorous gastric tissues [33]. STAT3 feedback regulation by SOCS3 is, in this case, attenuated, leading to increase in pSTAT3-induced cell proliferation and cell survival mechanisms (Fig. 2).
Tumour development and progression is being more often linked to tumour microenvironment and associated stromal cells [34, 35]. IL-6/JAK/STAT3 signalling seems to be involved in cancer associated fibroblasts-(CAFs) induced GC cells migration and epithelial to mesenchymal transition [36]. Here, JAK/STAT pathway serves as crosstalk between tumour cells and members of the associated microenvironment and stimulate GC progression.
The pro-tumorigenic effects (Fig. 2) of STAT3 in GC context described here mostly involve the GP130 subunit of the receptor, shared with other members of the IL-6 family of cytokines and demonstrate the role of IL-6 signalling in gastric carcinogenesis. However, LIF-mediated signalling which solicits GP190 has not been thoroughly studied in GC and the pleiotropic property of this cytokine makes it an interesting research point to dig in this context.
Induction of MAPK/ERK pathway by LIF leads to pro-proliferation capacities [14] and has been demonstrated as pro-tumorigenic in GC (Fig. 2). Similarly, AKT activation by LIF is found to have pro-proliferation, survival, or apoptotic roles. Pro-tumorigenic and pro-metastatic roles of LIF through AKT/mTOR pathway have been described in breast cancer [17] but have not been investigated in GC context. Despite the several signalling pathways known to be affected by LIF/LIFRβ, there is not much data assessing the role of LIF/LIFR signalling, independent of JAK/STAT3 in GC. Evidence from other types of cancer show that studying LIF/LIFR through other pathways could undoubtedly bring to light some beneficial aspects of LIF [19,20,21, 25].
Anti-tumorigenic aspects
Indeed, LIF appears to have a more positive side and its opposing effects in cancer seem to be related to the signalisation processes solicited [19, 21, 37]. LIFR has a tumour suppressor role in GC (Fig. 2). Zhang et al. show that LIFR is downregulated by miR-589, which is overexpressed in gastric tumour tissues and GC cells, compared to non-tumorous gastric tissues and cells [37]. miR-589 decreases LIFR inhibition of PI3K-AKT-c-Jun axis and contributes to cell migration, metastasis, and invasion (Fig. 2). miR-589 oncogene expression is in turn increased by c-Jun which activates its transcription by binding to its promoter. LIFR tumour suppressor action in GC was also reported by Zhao et al. who show that long coding RNA LOWEG can increase LIFR expression and decrease GC cells invasion capacity [38]. LOWEG is found to be under-expressed in GC tissues and cell lines compared to non-tumorous gastric tissue and cell lines, and its expression is positively correlated to that of LIFR.
Several studies make the Hippo pathway one possible cell signalisation process to be exploited in the LIF/LIFR/GC context. Cancer stem cells (CSCs) are a crucial aspect of gastric tumours, being at the origin of tumour initiation, resistance, and dissemination [39, 40] and their targeting is of utmost significance. The Hippo pathway’s importance in GC and cancer stem cells (CSCs) maintenance is a well-established fact today, making it a target of choice, and many studies involving other types of cancers relate Hippo/LIF/LIFR signalling connection. Chen et al. describes LIF/LIFR as upstream the Hippo pathway, participating to the anti-metastatic effects in breast cancer and LIFR-targeting by miR-9 as responsible for LIFR protein expression loss and associated metastasis [19]. Nandy et al. shows that miR-125a targets LIFR in breast cancer and in so doing, deregulates Hippo effector TAZ phosphorylation leading to increased tumorigenesis and CSCs maintenance [41]. Indeed, the Hippo pathway is made up of two opposing components: a tumour suppressor kinase core (MST1/2-LATS1/2) negatively phosphorylating a downstream block of oncogenic effectors (YAP/TAZ) which, when activated, can act as transcriptional co-factors for genes exacerbating CSCs’ role in tumour initiation, growth, dissemination and relapse contributing to the pro-tumorigenic and pro-CSC role of this pathway [40, 42,43,44]. In clear cell renal carcinoma, the authors also demonstrate that LIF/LIFR anti-invasive effects passes through a decrease in the expression of Hippo oncogenic effector YAP [21]. In the meanwhile, Guo et al. successfully verified that LIF/LIFR pro-metastatic effects in melanoma do not implicate the Hippo signalling pathway downstream LIF/LIFR, but the canonical pathway JAK/STAT showing the opposing effects of LIF/LIFR depending on the solicited pathway and confirming Hippo pathway study interest in the LIF/LIFR context [22]. Furthermore, the recent link between Hippo, GC tumorigenesis and gastric CSC makes LIF/LIFR/Hippo regulation study in GC even more attracting [42, 43].
In addition, both Ali et al. and Nandy et al. interestingly suggest a possible crosstalk between LIF/LIFR/STAT3 and LIF/LIFR/Hippo signalling and more a regulation of STAT3 downstream LIF/LIFR/Hippo since JAK/STAT3 inhibition does not change Hippo effectors level, but inhibition of Hippo effector TAZ phosphorylation leads to increased levels of STAT3 and pSTAT3 [18, 41]. Hippo pathway oncogenic effectors having transcriptional co-factor roles when activated, this suggests STAT3 as a possible target gene of Hippo oncogenic effectors and LIF/LIFR as negative regulator of this oncogenic core [19]. This appeals for more investigation on LIF/LIFR/Hippo/STAT3 and more specifically in the GC context where this has not been thoroughly inquired yet.
Nevertheless, among the few published studies exploiting LIF/LIFRβ signalling in GC, one shows LIFR as a tumour suppressor [38] and another that LIF can inhibit the proliferation of GC through a G1-phase arrest [45]. In the latter, authors show that LIF is under-expressed in GC and more specially in tissues with poorly differentiated GC cells and this is associated to poor prognosis of GC patients [45]. LIF overexpression in GC cells as well as recombinant LIF treatment upregulates p21 and decreases cyclin D1 expression and leads to delayed tumour progression in vivo [45]. This study again shows opposing effects of LIF/LIFR in cancer and though the mechanisms behind such an effect has not been investigated here, it arouses even more curiosity about LIF/LIFR potential effect and mechanism in GC and carves new paths and hypotheses for a possible anti-tumorigenic effect in this bad prognosis disease. In accordance with this, recent data from our team show that GC cell lines and patient-derived xenograft cells treatment with LIF is able to decrease gastric CSC population and properties in vitro by promoting the activity of Hippo pathway tumour suppressor kinase core and the JAK/STAT pathway does not seem to be implicated in the observed effects [44]. This hereby opens the field to more interesting research in this topic for LIF use in anti-CSC therapy, which is vital in GC where CSCs play a key role in tumour progression and relapse.
LIF: a new Gastric cancer therapeutic option?
LIF’s pleiotropy makes its possibility as therapy questionable. Nevertheless, in vivo attempts with LIF can be more and more found in literature (Table 1). LIF injection in mice has shown potential in cardiomyocyte regeneration [46], type 2 immunity suppression in Duchenne muscular atrophy [47] and Th17 accumulation inhibition for intestinal epithelium repair in inflammatory bowel disease [48]. Few clinical trials have also been carried out, notably the phase II trial of Davis et al. using Emfilermin, a commercial human LIF, for the prevention of peripheral neuropathy induced by chemotherapy [49]. Although LIF did not show any positive response on peripheral neuropathy in this context, it was noted that patients did not show any side effects corroborating the fact that LIF could potentially be used as a therapeutic drug in human. Several clinical trials have also been carried out by Merck kGaA and Rambam Health Care Campus for embryo implantation improvement in in vitro fertilisation processes [50,51,52,53,54]. Despite these clinical trials, caution must be exercised, and more tests are necessary, taking into account LIF’s high pleiotropy.
Furthermore, strategies have been developed for the targeted delivery of LIF, using as test LIF anti-proliferation capacity in murine leukemic M1 cells [55]. The authors conceived nanoparticles made of poly-ethylene glycol-poly(lactic acid), filled with LIF and coated with CD11b antibody allowing the targeting of activated peripheral macrophages, and capable of decreasing proliferation of M1 cells in vitro, giving hope for future use of LIF in targeted therapies, for example in the gastric CSC context. Despite its known anti-tumorigenic effects, no clinical trials using LIF in cancer therapy have been attempted yet, maybe due to its high pleiotropy.
Conclusion
LIF is indeed a remarkably interesting cytokine and LIF/LIFR signalling a complex process with all the different pathways which can be triggered or inhibited by LIFRβ-induced JAK phosphorylation. LIF/LIFR pleiotropy is highly related to the signalling pathways involved and further explorations might help elucidate LIF signalisation affinities depending on the cellular context. This review puts to light the complexity of LIF/LIFR biology in cancer and more particularly in GC. LIF/LIFRβ signalling has not been thoroughly investigated in the GC context, most studies involving IL-6/STAT3. This review relates the pro- and anti-tumorigenic aspects of LIF/LIFRβ signalling in GC. LIF, especially in a STAT3-independent context, seems to be promising in terms of anti-GC effects and therapy.
References
Yue X, Wu L, Hu W. The regulation of leukemia inhibitory factor. Cancer Cell Microenviron. 2015;2:e877.
Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, et al. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987;6:3995–4002.
Smith AG, Nichols J, Robertson M, Rathjen PD. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev Biol. 1992;151:339–51.
Moreau JF, Donaldson DD, Bennett F, Witek-Giannotti J, Clark SC, Wong GG. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature. 1988;336:690–2.
Rosario GX, Stewart CL. The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol. 2016;75:246–55.
Oshima K, Teo DTW, Senn P, Starlinger V, Heller S. LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol. 2007;7:112.
Jia D, Cai M, Xi Y, Du S, Zhenjun T. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018;193:77–86.
Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015;26:533–44.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48.
Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46.
Giese B, Roderburg C, Sommerauer M, Wortmann SB, Metz S, Heinrich PC, et al. Dimerization of the cytokine receptors gp130 and LIFR analysed in single cells. J Cell Sci. 2005;118:5129–40.
Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83.
Pinho V, Fernandes M, da Costa A, Machado R, Gomes AC. Leukemia inhibitory factor: Recent advances and implications in biotechnology. Cytokine Growth Factor Rev. 2020;52:25–33.
Kershaw NJ, Murphy JM, Liau NPD, Varghese LN, Laktyushin A, Whitlock EL, et al. SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat Struct Mol Biol. 2013;20:469–76.
Kershaw NJ, Laktyushin A, Nicola NA, Babon JJ. Reconstruction of an active SOCS3-based E3 ubiquitin ligase complex in vitro: identification of the active components and JAK2 and gp130 as substrates. Growth Factors. 2014;32:1–10.
Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C, et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget. 2014;5:788–801.
Ali SA, Kaur G, Kaushik JK, Malakar D, Mohanty AK, Kumar S. Examination of pathways involved in leukemia inhibitory factor (LIF)-induced cell growth arrest using label-free proteomics approach. J Proteom. 2017;168:37–52.
Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–7.
Luo Q, Wang C, Jin G, Gu D, Wang N, Song J, et al. LIFR functions as a metastasis suppressor in hepatocellular carcinoma by negatively regulating phosphoinositide 3-kinase/AKT pathway. Carcinogenesis. 2015;36:1201–12.
Lei C, Lv S, Wang H, Liu C, Zhai Q, Wang S, et al. Leukemia inhibitory factor receptor suppresses the metastasis of clear cell renal cell carcinoma through negative regulation of the yes-associated protein. DNA Cell Biol. 2018;37:659–69.
Guo H, Cheng Y, Martinka M, McElwee K. High LIFr expression stimulates melanoma cell migration and is associated with unfavorable prognosis in melanoma. Oncotarget. 2015;6:25484–98.
Buckley AM, Lynam-Lennon N, Kennedy SA, Dunne MR, Aird JJ, Foley EK, et al. Leukaemia inhibitory factor is associated with treatment resistance in oesophageal adenocarcinoma. Oncotarget. 2018;9:33634–47.
Gulluoglu S, Sahin M, Tuysuz EC, Yaltirik CK, Kuskucu A, Ozkan F, et al. Leukemia inhibitory factor promotes aggressiveness of chordoma. Oncol Res. 2017;25:1177–88.
Ma D, Jing X, Shen B, Liu X, Cheng X, Wang B, et al. Leukemia inhibitory factor receptor negatively regulates the metastasis of pancreatic cancer cells in vitro and in vivo. Oncol Rep. 2016;36:827–36.
Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569:131–5.
Terawaki K, Sawada Y, Kashiwase Y, Hashimoto H, Yoshimura M, Suzuki M, et al. New cancer cachexia rat model generated by implantation of a peritoneal dissemination-derived human stomach cancer cell line. Am J Physiol Endocrinol Metab. 2014;306:E373-387.
Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–78.
Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073–85.
Jackson CB, Judd LM, Menheniott TR, Kronborg I, Dow C, Yeomans ND, et al. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140–51.
Fukui H, Watari J, Zhang X, Ran Y, Tomita T, Oshima T, et al. Phosphorylated STAT3 expression linked to SOCS3 methylation is associated with proliferative ability of gastric mucosa in patients with early gastric cancer. Oncol Lett. 2020;19:3542–50.
Zhang X-M, Zhou C, Gu H, Yan L, Zhang G-Y. Correlation of RKIP, STAT3 and cyclin D1 expression in pathogenesis of gastric cancer. Int J Clin Exp Pathol. 2014;7:5902–8.
Xiao C, Hong H, Yu H, Yuan J, Guo C, Cao H, et al. MiR-340 affects gastric cancer cell proliferation, cycle, and apoptosis through regulating SOCS3/JAK-STAT signaling pathway. Immunopharmacol Immunotoxicol. 2018;40:278–83.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22.
Haykal MM, Nahmias C, Varon C, Martin OCB. Organotypic modeling of the tumor landscape. Front Cell Dev Biol. 2020;8:606039.
Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741–50.
Zhang F, Li K, Pan M, Li W, Wu J, Li M, et al. miR-589 promotes gastric cancer aggressiveness by a LIFR-PI3K/AKT-c-Jun regulatory feedback loop. J Exp Clin Cancer Res. 2018;37:152.
Zhao J-H, Sun J-X, Song Y-X, Chen X-W, Yang Y-C, Ma B, et al. A novel long noncoding RNA-LOWEG is low expressed in gastric cancer and acts as a tumor suppressor by inhibiting cell invasion. J Cancer Res Clin Oncol. 2016;142:601–9.
Nguyen PH, Giraud J, Chambonnier L, Dubus P, Wittkop L, Belleannée G, et al. Characterization of biomarkers of tumorigenic and chemoresistant cancer stem cells in human gastric carcinoma. Clin Cancer Res. 2017;23:1586–97.
Seeneevassen L, Bessède E, Mégraud F, Lehours P, Dubus P, Varon C. Gastric cancer: advances in carcinogenesis research and new therapeutic strategies. Int J Mol Sci. 2021;22:3418.
Nandy SB, Arumugam A, Subramani R, Pedroza D, Hernandez K, Saltzstein E, et al. MicroRNA-125a influences breast cancer stem cells by targeting leukemia inhibitory factor receptor which regulates the Hippo signaling pathway. Oncotarget. 2015;6:17366–78.
Molina Castro SE, Tiffon C, Giraud J, Boeuf H, Sifre E, Giese A, et al. The Hippo kinase LATS2 controls Helicobacter pylori-induced epithelial-mesenchymal transition and intestinal metaplasia in gastric mucosa. Cell Mol Gastroenterol Hepatol. 2020;9:257–76.
Giraud J, Molina-Castro S, Seeneevassen L, Sifré E, Izotte J, Tiffon C, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer. 2019;146:2255–67.
Seeneevassen L, Giraud J, Molina-Castro S, Sifré E, Tiffon C, Beauvoit C, et al. Leukaemia inhibitory factor (LIF) inhibits cancer stem cells tumorigenic properties through hippo kinases activation in gastric cancer. Cancers (Basel). 2020;12:2011.
Xu G, Wang H, Li W, Xue Z, Luo Q. Leukemia inhibitory factor inhibits the proliferation of gastric cancer by inducing G1-phase arrest. J Cell Physiol. 2019;234:3613–20.
Kanda M, Nagai T, Takahashi T, Liu ML, Kondou N, Naito AT, et al. Leukemia inhibitory factor enhances endogenous cardiomyocyte regeneration after myocardial infarction. PLoS ONE. 2016;11: e0156562.
Welc SS, Flores I, Wehling-Henricks M, Ramos J, Wang Y, Bertoni C, et al. Targeting a therapeutic LIF transgene to muscle via the immune system ameliorates muscular dystrophy. Nat Commun. 2019;10:2788.
Zhang YS, Xin DE, Wang Z, Song X, Sun Y, Zou QC, et al. STAT4 activation by leukemia inhibitory factor confers a therapeutic effect on intestinal inflammation. EMBO J. 2019;38: e99595.
Davis ID, Kiers L, MacGregor L, Quinn M, Arezzo J, Green M, et al. A randomized, double-blinded, placebo-controlled phase II trial of recombinant human leukemia inhibitory factor (rhuLIF, emfilermin, AM424) to prevent chemotherapy-induced peripheral neuropathy. Clin Cancer Res. 2005;11:1890–8.
Rambam Health Care Campus. Comparison of LIF (Leukemia Inhibitory Factor) Level Between Neonates Who Are IUGR (Intra Uterine Growth Restriction) and Those Who Are AGA (Average for Gestational Age) [Internet]. clinicaltrials.gov; 2015 Aug. Report No.: NCT02518126. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02518126
Rambam Health Care Campus. The Correlation Between LIF Levels in Cord Blood to Maternal Blood in Women Treated With Mg During Labor [Internet]. clinicaltrials.gov; 2015 Aug. Report No.: NCT02507817. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02507817
Merck KGaA, Darmstadt, Germany. A Randomised, Double-blind, Placebo Controlled, Proof of Concept Study to Assess the Efficacy, Safety and Acceptability of r-hLIF for Improving Embryo Implantation Following in Vitro Fertilisation (IVF) and Embryo Transfer (ET) in Women With Recurrent Implantation Failure. [Internet]. clinicaltrials.gov; 2017 Jan. Report No.: NCT00504530. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00504530
Merck KGaA, Darmstadt, Germany. A Multicentre, Randomised, Double-blind, Placebo-controlled Proof of Concept Study to Compare the Efficacy and Safety of r-hLIF (Emfilermin) for Improving Embryo Implantation Following in Vitro Fertilization (IVF) and Embryo Transfer (ET) in Women With Recurrent Implantation Failure [Internet]. clinicaltrials.gov; 2017 Jan. Report No.: NCT00504608. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00504608
MD YG. The Correlation Between Gestational Age and Maternal and Fetal Levels of LIF and ACTH [Internet]. clinicaltrials.gov; 2017 Aug. Report No.: NCT03231904. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03231904
Davis SM, Reichel D, Bae Y, Pennypacker KR. Leukemia inhibitory factor-loaded nanoparticles with enhanced cytokine metabolic stability and anti-inflammatory activity. Pharm Res. 2018;35:6.
Acknowledgements
The PhD fellowship of Lornella Seeneevassen was funded by the French Ministry of Tertiary Education, Research and Innovation. This work was funded by the Ligue Française Contre le Cancer/French League against Cancer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seeneevassen, L., Martin, O.C.B., Lehours, P. et al. Leukaemia inhibitory factor in gastric cancer: friend or foe?. Gastric Cancer 25, 299–305 (2022). https://doi.org/10.1007/s10120-022-01278-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01278-w