Abstract
Background
Gastric cancer (GC) patients with peritoneal metastasis are defined as stage IV in the Japanese classification of GC. For patients with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) and/or localized peritoneal metastasis (P1a), gastrectomy followed by S1 monotherapy is one of the most widely accepted therapeutic strategy in Japan. This study investigated the efficacy of preoperative chemotherapy as initial treatment in GC patients with CY1 and/or P1a.
Methods
We retrospectively reviewed GC patients diagnosed with CY1 and/or P1a at 34 institutions in Japan between 2008 and 2012. Selection criteria were: adenocarcinoma, no distant metastasis except CY1 or P1a, and no prior treatment. The subjects were divided into an Initial-Chemotherapy group and an Initial-Surgery group, according to the initial treatment.
Results
A total of 824 patients were collected and 713 eligible patients were identified for this study. As the initial treatment, 150 patients received chemotherapy (Initial-Cx), and 563 patients underwent surgery (Initial-Sx). Initial-Cx regimens were cisplatin plus S1/docetaxel plus cisplatin plus S1/others (n = 90/37/23). Both overall survival (OS) and progression-free survival (PFS) were similar between the Initial-Cx and Initial-Sx groups (median OS 24.8 and 24.0 months, HR 1.07, 95% CI 0.87–1.3; median PFS 14.9 and 13.9 months, HR 1.04, 95% CI 0.85–1.27). The 5-year OS rates were 22.3% in the Initial-Cx group and 21.5% in the Initial-Sx group.
Conclusions
Although, the preoperative chemotherapy did not show a survival benefit for GC patients with CY1 and/or P1a, initial-Cx showed favorable survival in patients who converted to P0 and CY0.
Similar content being viewed by others
Introduction
The peritoneum is one of the most frequent metastatic sites of gastric cancer (GC). Peritoneal lavage cytology is a useful method for detecting peritoneal dissemination even in patients without visible metastatic disease, and its positivity is an important predictive factor of peritoneal recurrence and poor prognosis in GC [1,2,3]. Therefore, cytological examination of peritoneal lavage is recommended by the Japanese Gastric Cancer Treatment Guidelines, and positive peritoneal lavage cytology (CY1) is defined as a metastatic (M1) factor for staging in the 15th edition of the Japanese Classification of Gastric Carcinoma [4]. Similarly, peritoneal metastasis localized at a limited area close to the primary tumor is defined as P1a [5]. In a previous report, the prognosis of patients with P1a after surgical resection of all visible disease was reported to be similar to that of patients with CY1 [6, 7].
Systemic chemotherapy has been widely accepted as standard therapy for stage IV GC patients globally. However, gastrectomy with lymph node dissection leaving no visible disease followed by S1 monotherapy is one of the most widely accepted therapeutic strategy for GC patients with CY1 and/or P1a in clinical practice, in Japan. Because previous reports suggested that post-operative chemotherapy of S-1 monotherapy would prolong the survival for the patients with CY1 and/or P1a. It was reported 5-year overall survival (OS) rate of surgery alone was 7.8% [1], while 5-year overall survival (OS) rates of surgery followed by S-1 monotherapy was 20–30% [8,9,10,11]. However, their prognosis is still poor.
On the other hand, it was reported that systemic chemotherapy could eliminate the limited metastatic disease in some patients, converting to resectable disease and achieving long-term survival after curative surgery [12,13,14]. Based on these previous reports, it is considered that preoperative chemotherapy as initial treatment for GC patients with CY1 and/or P1a would be a promising treatment strategy, and it has been attempted recently in some Japanese institutions. However, few reports have evaluated the efficacy of preoperative chemotherapy as initial treatment for GC patients with CY1 and/or P1a.
This study investigated the efficacy of preoperative chemotherapy as initial treatment in GC patients with CY1 and/or P1a. This retrospective study compared the efficacy between initial chemotherapy followed by surgery and initial surgery followed by chemotherapy for GC patients with CY1 and/or P1a.
Materials and methods
Patients
We retrospectively reviewed the medical records of GC patients who were diagnosed with CY1 and/or P1a before initial treatment at 34 institutions in Stomach Cancer Group of the Japan Clinical Oncology Group (JCOG) between 2008 and 2012. We selected the patients who met the following selection criteria: age > 20 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2, histological diagnosis of gastric adenocarcinoma, CY1 (positive peritoneal lavage cytology) and/or P1a (metastasis to peritoneal surfaces adjacent to the stomach limited to the area above the transverse colon or the omentum) diagnosed by staging laparoscopy or laparotomy, no distant metastasis other than CY1 or P1a, and no prior treatment for GC.
Treatment and procedure
The diagnostic procedure of CY1 or P1a was entrusted to each institution. The treatment procedure was decided by each physician, such as initial treatment (chemotherapy or surgery), indication of chemotherapy (pre- and/or postoperative), chemotherapy regimens, duration of chemotherapy, and indication of surgery and surgical procedures.
Evaluation and statistical analysis
The patients selected for this study were divided into two groups: Initial-Chemotherapy (Initial-Cx) group and Initial-Surgery (Initial-Sx) group. The Initial-Cx group included patients who received chemotherapy as the initial treatment, while the Initial-Sx group comprised patients who underwent surgery as the initial treatment. Re-staging after the initial Cx was evaluated by each physician using CT scans and other methods, and surgery was performed if there were no progressions. The final staging after th initial-Cx, including the therapeutic effect on CY1 and P1a, was diagnosed as a result of the surgery.
We compared OS and progression-free survival (PFS) between the Initial-Cx group and the Initial-Sx group. OS was calculated from the date of diagnosis of CY1 and/or P1a, to the date of death from any cause or censored at the last visit. Disease progression was assessed by image examination according to the RECIST ver. 1.1, and PFS was calculated from the date of diagnosis of CY1 and/or P1a, to the date of progression or death from any cause, and surviving patients without disease progression were censored at the visit. OS and PFS were estimated using the Kaplan–Meier method and compared by the log-rank test.
To adjust for the patients’ background, survival differences among the treatment groups were evaluated by multivariate analyses using the Cox proportional hazard regression model, and presented as the hazard ratio (HR) and 95% CI. Covariates for the multivariate analysis included the initial treatment (initial Cx vs. initial Sx), peritoneal metastasis (P0 vs. P1a), peritoneal lavage cytology (CY0 vs. CY1), age (≤ 65 vs. > 65 years), ECOG PS, cT stage, and cN stage. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). p values < 0.05 were considered to denote statistically significant differences.
Results
Patient characteristics
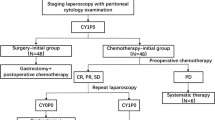
Data on a total of 824 patients were collected. Figure 1 presents a CONSORT diagram of the study population. Of the 713 selected patients, 150 received chemotherapy as initial treatment (Initial-Cx group) and 563 underwent surgery as initial treatment (Initial-Sx group). Their characteristics are shown in Table 1. Baseline characteristics were similar between the two groups. Median age in the Initial-Cx group was younger than that in the Initial-Sx group (Initial-Cx: 63 years, Initial-Sx: 67 years). The proportions of P0CY1/P1aCY0/P1aCY1 were 68.7/12/19.3% in Initial-Cx and 67.9/17.9/14.2% in Initial-Sx, respectively. Notably, CY1 and/or P1a before the initial treatment were diagnosed by staging laparoscopy and laparotomy in 136 (91%) and 14 (9%) patients in the initial-Cx group and in 61 (11%) and 502 (89%) patients in the initial-Sx group.
In the Initial-Cx group, the chemotherapy regimens were cisplatin plus S1 (CS; n = 90), docetaxel plus cisplatin plus S1 (DCS; n = 37), and others (n = 23). The details of the regimens in the Initial-Cx group are listed in Table 2. The median treatment duration of Initial-Cx was 62 days (range 36–884) in the CS regimen, 79 days (range 22–224) in the DCS regimen, and 35 days (range 14–158) in the other regimens. Median duration between staging laparoscopy and initial chemotherapy was 14 days (range 6–45). The surgical data are summarized in Table 3. Among the 150 patients in the Initial-Cx group, 110 (74%) underwent gastrectomy and 97 (64.7%) achieved R0/1 resection leaving no visible disease. After R0/1 resection, 89 patients received postoperative chemotherapy with S-1 monotherapy (n = 60), cisplatin plus S-1 (n = 6), and others (n = 23). The median follow-up time was 70.2 months (range 3.8–96.4). Among the 40 patients who did not receive gastrectomy after initial Cx, 28 received palliative chemotherapy and 12 did not.
In the Initial-Sx group, 506 patients (89%) underwent R0/1 resection and 444 (79%) received postoperative chemotherapy with S-1 monotherapy (n = 267), cisplatin plus S-1 (n = 114), or others (n = 63). The median treatment duration of postoperative chemotherapy was 285 days (range 2–2191) for S-1, 170 days (range 10–1774) for CS, and 223 days (range 7–1255) for the other regimens. The median duration of follow-up was 61.4 months (range 0.4–107.1).
Progression-free survival and overall survival
There was no statistically significant difference in OS between the two groups (HR 1.07, 95% CI 0.87–1.32, p = 0.502). The median OS was 24.8 months (95% CI 20.7–29.8) in the Initial-Cx group and 24.0 months (95% CI 21.7–26.3) in the Initial-Sx group. The 5-year OS rates were 22.3% in the Initial-Cx group and 21.5% in the Initial-Sx group (Fig. 2a).
The durations of PFS were also similar between the two groups (HR 1.04, 95% CI 0.85–1.27, p = 0.694). The median PFS was 14.9 months (95% CI 11.5–18.3) in the Initial-Cx group and 13.9 months (95% CI 12.2–15.4) in the Initial-Sx group. The 5-year PFS rates were 16.1% in the Initial-Cx group and 15.8% in the Initial-Sx group (Fig. 2b).
Multivariate analysis of OS did not show significant differences between the Initial-Sx group and Initial-Cx group (HR 1.103, 95% CI 0.892–1.365, p = 0.365). The over 65 years was identified as independent prognostic factor for OS (p < 0.05) (Table 4).
Subgroup analysis in the Initial-Chemotherapy group
Conversion to P0 and CY0 after initial Cx was obtained in 57 (38.0%) of all 150 patients in the Initial-Cx group: 19 (51.4%) of 37 patients treated with the DCS regimen, 34 (37.8%) of 90 patients with the CS regimen, and 4 (17.4%) of the 23 patients with the other regimens (Table 5). The patients who converted to P0 and CY0 after initial Cx showed better survival than those who did not (median OS, 32.0 vs. 18.8 months, HR = 2.04, 95% CI 1.37–3.03, p = 0.001) (Fig. 3a). In contrast, among the 93 patients who did not convert to P0 and CY0 after initial Cx, 40 patients who did not undergo surgery showed worse survival than those who did (median OS, 24.6 vs. 12.5 months, HR = 2.61, 95% CI 1.62–4.20, p = 0.001). In terms of the median OS in patients who did not convert to P0 and CY0 and underwent surgery, this was 34.9 months in the R0/1 resection group and 17.2 months (n = 40, 95% CI 21–51.3) in the R2 resection group (n = 13, 95% CI 8–20.7). The median OS in patients with negative or non-negative conversion was 27.0 (n = 45, 95% CI 23.5–48.9) and 21.0 (n = 59, 95% CI 14.8–30.1) months in the P0CY1 group (HR 0.68, 95% CI 0.42–1.08, p = 0.11); not reached (NR) (n = 5, 95% CI 42.2–NR) and 22.5 months (n = 13, 95% CI 20.7–51.1) in the P1CY0 group (HR 0.07, 95% CI 0.003–0.38, p = 0.001); and 42.8 (n = 7, 95% CI 15.7–NR) and 12.5 months (n = 21, 95% CI 11.1–31.7) in the CY1P1a group (HR 0.32, 95% CI 0.11–0.77, p = 0.01), respectively. The median OS was 27.0 months (95% CI 19.9–70.6) for DCS, 23.5 months (95% CI 20.2–30.1) for CS, and 18.7 months (95% CI 11.5–35.5) for the other regimens (Fig. 3b). Although there were no statistically significant differences in OS among the three initial-Cx, DCS tended to show better OS than the other two groups (DCS vs. CS: HR 0.70, 95% CI 0.43–1.10, p = 0.139; DCS vs. others: HR 0.58, 95% CI 0.32–1.08, p = 0.078).
Subgroup analysis. a Kaplan–Meier analysis curve for overall survival of patients who converted to P0 CY0 or not after initial Cx. b Kaplan–Meier analysis curve for overall survival according to treatment group in initial Cx. c Kaplan–Meier analysis curves for overall survival of patients who converted to P0 CY0 after initial Cx and patients in the Initial-Sx group
Subgroup analysis in the Initial-Surgery group
In terms of the median OS in the Initial-Sx group, this was 25.0 months (95% CI 19.9–70.6) in the R0/1 resection group and 18.8 months (95% CI 20.2–30.1) in the R2 resection group. Median OS stratified by the postoperative chemotherapy in the Initial-Sx group (R0/1 resection) was 29.5 months (95% CI 25.2–32.9) in the S-1 group, 24.7 months (95% CI 20.6–29.6) in the CS group, 25.4 months (95% CI 18.8–38.4) in the ‘others’ group, and 9.9 months (95% CI 6.6–12.8) in the no-Cx group.
The patients who converted to P0 and CY0 after initial-Cx showed better survival than Initial-Sx group (median OS, 32.0 vs. 24.0 months, HR = 1.64, 95% CI 1.17–2.31, p = 0.004) (Fig. 3c).
Discussion
While gastrectomy followed by S1 therapy is one of the most widely accepted therapeutic strategy of GC patients with CY1 and/or P1a in Japan, the Initial-Sx group in this study provided real-world data of Japanese clinical practice. Among the previous reports, 5-year survival rates of gastrectomy followed by S-1 monotherapy for GC patients with CY1 and/or P1a were 20–30% [8,9,10,11]. In this study, 5-year OS rates were 22.3% in the Initial-Cx group and 21.5% in the Initial-Sx group. These data support the consistency of 5-year OS rates in this population, meaning that GC patients with CY1 and/or P1a can be a target for cure. It seems reasonable to consider that these data could be adopted as a consistent historical control when we conduct a clinical trial for GC patients with CY1 and/or P1a in the future.
In East Asia, since the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) showed a survival benefit of adjuvant chemotherapy with S-1 alone compared with surgery alone, there has been some progress achieved using doublet adjuvant chemotherapy such as capecitabine plus oxaliplatin and S-1 plus docetaxel [15, 16]. However, in our previous report, there was no difference in OS between S-1 alone and doublet chemotherapy as postoperative chemotherapy for gastric cancer patients with CY1 and/or P1a [11]. Therefore, while there were some variations of chemotherapy regimens of the Initial-Sx group, especially in postoperative chemotherapy, it is considered that post-chemotherapy regimens would have similar impact on OS, regardless of the regimens.
Considering the survival impact of gastrectomy, among the 93 patients who did not convert to P0 and CY0 after initial Cx, the median OS of the 40 patients not receiving gastrectomy for removal of all visible disease was as short as 12.9 months, which is similar to that reported in a clinical trial of palliative first-line chemotherapy for advanced gastric cancer [17,18,19]. In contrast, that of the other 53 patients who received gastrectomy resulting in no visible disease was 24.6 months, which is similar to that in the Initial-Sx group. Although there should be some bias regarding the decision of surgery under the treatment policy for the indication of gastrectomy depending on each institution, there was a substantial difference in OS according to surgery (HR 2.61, 95% CI 1.62–4.20, p = 0.001). In the REGATTA trial, gastrectomy leaving non-curative factors did not show a survival benefit compared with chemotherapy alone for advanced gastric cancer patients with a single non-curative factor) [20]. However, the treatment goal depending on the remaining tumor volume differs quite substantially between our study and the REGATTA trial: a curative setting leaving no visible tumor in our study vs. a palliative setting leaving a non-curative factor in the REGATTA trial. These results suggest that gastrectomy leaving no visible disease might have some impact on survival.
Interestingly, patients who achieved P0 and CY0 after receiving initial Cx showed favorable survival. Moreover, the DCS regimen showed the highest rate of conversion to P0 and CY0 and longer survival than other regimens. These results suggest that the initial-Cx regimen with greater tumor shrinkage effects may contribute to longer survival and a higher curative rate of GC patients with CY1 and/or P1. As for cytotoxic agents, a triplet regimen consisted of FU, platinum, and taxane is one of the most promising regimens showing a higher response rate than doublet chemotherapy. S-1 plus leucovorin and oxaliplatin is another promising regimen, because it showed a higher response rate (75%) than those reported in other phase III trials of advanced gastric cancer [21, 22]. The combination of these cytotoxic agents with new agents in other classes, such as immune-checkpoint inhibitors and molecular-targeted agents, is also a promising approach for future progress [23, 24]. As for the treatment modality, intraperitoneal chemotherapy (IP) is also a promising therapeutic approach [25]. A phase III study, the PHOENIX-GC trial, evaluated the superiority of intraperitoneal paclitaxel plus systemic chemotherapy (IP) relative to the standard chemotherapy (SP). Although it failed to show a survival benefit of IP due to its small sample size, the median survival times for the IP and SP arms were 17.7 and 15.2 months, respectively (HR 0.72, 95% CI 0.49–1.04, p = 0.08) [26]. Moreover, the proportion of conversion to CY0 was reported to be as high as 76% in the IP group (69 out of 91 patients). Based on our results that patients achieving conversion to CY0 P0 showed favorable survival, it is expected that IP chemotherapy achieving a higher proportion of conversion to CY0 IP may be another promising therapeutic approach for GC patients with CY0 and/or P1a.
This study has several limitations. First, this was a retrospective study and no established standard operation for CY1 / P1a GC patients, which might contain some bias regarding determination of the investigator's treatment policy for each patient: initial Cx or initial Sx. Second, the diagnosis of CY1 or P1a, most of the cases in the Initial-Sx group were diagnosed at the time of laparotomy, and most of the cases in the Initial-Cx group were diagnosed at the time of staging laparoscopy. However, the study do not collect detailed information on the CY or P1a diagnosis process. Although we adjusted for well-known prognostic factors, other potential prognostic factors may have had some influence on the outcomes. Meanwhile, the treatment strategy, Initial-Cx or Initial-Sx, depended mainly on the policy of each hospital, and there were no major differences in patient background such as the tumor burden (CY1 and/or P1a) and PS between the Initial-Cx and Initial-Sx groups. Moreover, the postoperative chemotherapy in this study might be out of date, considering the new evidence of adjuvant chemotherapy for curatively resected GC. Furthermore, data about toxicities and quality of life were not collected.
In conclusion, the preoperative chemotherapy did not show a survival benefit for GC patients with CY1 and/or P1a. However, initial Cx showed favorable survival in patients who converted to P0 and CY0. Further development of a novel preoperative chemotherapeutic regimen or innovative treatment strategy that targets peritoneal metastasis is required to improve the survival of GC patients with CY1 and/or P1a.
References
Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62.
Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, Miyata H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204.
Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol. 1978;22:225–9.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 3rd English edition. Gastric Cancer. 2011;14:101–12.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2020). https://doi.org/10.1007/s10120-020-01042-y.
Cabalag CS, Chan ST, Kaneko Y, Duong CP. A systematic review and meta-analysis of gastric cancer treatment in patients with positive peritoneal cytology. Gastric Cancer. 2015;18(1):11–22.
Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Kobayashi D, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–5.
Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, et al. Long-term follow up of patients who were positive for peritoneal lavage cytology:final report from the CCOG0301 study. Gastric Cancer. 2012;15(3):335–7.
Kano Y, Kosugi S, Ishikawa T, Otani T, Muneoka Y, Sato Y, et al. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery. 2015;158(6):1581–9.
Nakayama I, Chin K, Matsushima T, Takahari D, Takahari D, Ogura M, Shinozaki E, et al. Retrospective comparison of S-1 plus cisplatin versus S-1 monotherapy for the treatment of advanced gastric cancer patients with positive peritoneal cytology but without gross peritoneal metastasis. Int J Clin Oncol. 2017;22:1060–8.
Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, et al. Efficacy of postoperative chemotherapy after resection that leaves no macroscopically visible disease of gastric cancer with positive peritoneal lavage cytology (CY1) or localized peritoneum metastasis (P1a): a multicenter retrospective study. Ann Surg Oncol. 2020;27(1):284–92.
Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16(12):3227–36.
Okabe H, Hata H, Hosogi H, Ueda S, Ota S, Kinjo Y, et al. A phase 2 study of induction chemotherapy using docetaxel, cisplatin, and S1 for gastric cancer with peritoneal metastasis (KUGC06). Ann Surg Oncol. 2019;26(6):1779–86.
Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. 2012;15(1):61–9.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–21.
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10(11):1063–9.
Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N, et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(7):501–10.
Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–18.
Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):99–108.
Kang Y, Chin K, Chung H, Kadowaki S, Oh S, Nakayama N, et al. A phase III study of TAS-118 plus oxaliplatin versus S-1 plus cisplatin as first-line chemotherapy in patients with advanced gastric cancer (SOLAR study). Ann Oncol. 2019;30(Suppl 4):iv 153.
Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30(2):250–8.
Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, et al. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97–106.
Naoto T, Mitsuro K, Takaki Y, Takiguchi N, Fujitani K, Miyamoto K, et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: final results of the INPACT trial. Gastric Cancer. 2018;21:1014–23.
Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36(19):1922–9.
Acknowledgements
We thank all of the participating patients and the participating co-investigators, for their cooperation in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Atsuo Takashima received a research grant from Taiho and Ono, and honoraria from Taiho, Ono, and Bristol-Myers Squibb, outside the submitted work. Dr. Kengo Nagashima reports personal fees from Pfizer R&D Japan G.K., and personal fees from Toray Industries, Inc., outside the submitted work. Dr. Masanori Terashima reports personal fees from Taiho, personal fees from Chugai, personal fees from Ono, personal fees from BMS, personal fees from Yakult, personal fees from Takeda, personal fees from Eli Lilly, personal fees from Pfizer, and personal fees from Daiichi Sankyo, outside the submitted work. Dr. Kazuhiro Nishikawa reports personal fees from Chugai, personal fees from Taiho, personal fees from Yakult, personal fees from Eli Lilly, personal fees from EA Pharma, personal fees from Ono, and personal fees from Bristol-Myers Squibb, outside the submitted work. Dr. Satoh reports grants, personal fees from Ono Pharmaceutical, grants, personal fees, and other from Chugai Pharmaceutical, grants, personal fees, and other from Yakult Honsha, grants and personal fees from Eli Lilly, grants and personal fees from MSD, grants from Giliad Sciences, grants from Palexell, grants and personal fees from Bristol Myers Squib, grants and personal fees from Astellas, grants from Daiichi Sankyo, grants and personal fees from Taiho Pharmaceutical, personal fees from TakaraBio, and personal fees from Sanofi-Aventis, outside the submitted work. Dr. Narikazu Boku received research grants from Taiho and Ono, and honoraria from Taiho, Ono, and Bristol-Myers Squibb, outside the submitted work. All remaining authors have declared no conflicts of interest.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Takashima, A., Nagashima, K. et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. Gastric Cancer 24, 701–709 (2021). https://doi.org/10.1007/s10120-020-01137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01137-6