Abstract
Background
This study aimed to investigate risk factors for lymph node (LN) or distant metastasis after non-curative endoscopic resection (ER) of undifferentiated-type early gastric cancer (EGC).
Methods
Of 1124 patients who underwent ER for undifferentiated-type gastric cancer at 18 tertiary hospitals across six geographic areas in Korea between 2005 and 2014, 634 with non-curative ER beyond the expanded criteria were retrospectively enrolled. According to the treatment after ER, patients were divided into additional surgery (n = 270) and follow-up (n = 364) groups. The median follow-up duration was 59 months for recurrence and 84 months for mortality.
Results
LN metastasis was found in 6.7% (18/270) of patients at surgery. Ulcer [odds ratio (OR) 3.83; 95% confidence interval (CI) 1.21–12.13; p = 0.022] and submucosal invasion (OR 10.35; 95% CI 1.35–79.48; p = 0.025) were independent risk factors. In the follow-up group, seven patients (1.9%) developed LN or distant recurrence. Ulcer [hazard ratio (HR) 7.60; 95% CI 1.39–35.74; p = 0.018], LVI (HR 6.80; 95% CI 1.07–42.99; p = 0.042), and positive vertical margin (HR 6.71; 95% CI 1.28–35.19; p = 0.024) were independent risk factors. In the overall cohort, LN metastasis rates were 9.6% in patients with two or more risk factors and 1.2% in those with no or one risk factor.
Conclusions
LVI, ulcer, submucosal invasion, and positive vertical margin are independently associated with LN or distant metastasis after non-curative ER of undifferentiated-type EGC. Surgical resection is strongly recommended for patients with two or more risk factors.
Similar content being viewed by others
Introduction
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer mortality worldwide, accounting for > 1 million new cases and 783,000 deaths in 2018 [1]. Gastric cancer detected in the early stage has a good prognosis. In particular, for early gastric cancer (EGC) with no lymph node (LN) metastasis (stage IA), the 5-year overall survival rate is 95% [2]. Endoscopic resection (ER) provides excellent long-term survival, comparable to that provided by surgery, if curative resection with a minimal risk of LN metastasis is achieved [3]. When resection is considered non-curative beyond the expanded criteria, additional surgical resection with LN dissection is recommended because of the potential risk of LN metastasis [4, 5].
The rate of LN metastasis ranges between 5.1 and 12.2% after non-curative ER [6,7,8,9,10]. In clinical practice, a considerable proportion of patients choose simple follow-up without surgery for various reasons. Thus, in previous studies, lymphovascular invasion (LVI) and deep submucosal invasion were identified as risk factors for LN or distant metastasis, which were either found during surgery or detected during follow-up, and accordingly, radical surgery was strongly recommended for patients with these risk factors [7,8,9,10]. These studies mainly focused on the differentiated-type EGC. Undifferentiated-type EGC, however, has different risk factors for LN metastasis. Tumor size > 2 cm or submucosal invasion < 500 μm from the muscularis mucosa have been associated with an increased risk of LN metastasis [11]; however, previous studies did not investigate these specific criteria for undifferentiated-type cancers. In addition, risk factors of mortality may also be important for patients who are followed up without surgery after non-curative ER because these patients may not undergo detailed surveillance examinations. A large-scale study showed that LVI was associated with increased mortality in patients with differentiated-type EGC who underwent non-curative ER but were followed up without surgery [12]. It is not clear whether this finding is also applicable to undifferentiated-type cancer.
Therefore, we conducted a nationwide multicenter cohort study to investigate the risk factors of LN or distant metastasis and mortality after non-curative ER of undifferentiated-type EGC, in order to determine whether surgery can be strongly recommended for patients with those risk factors.
Methods
Patients
This retrospective cohort study included consecutive patients who underwent non-curative ER beyond the expanded criteria for undifferentiated-type EGC at 18 tertiary hospitals across 6 geographic areas in Korea between January 2005 and December 2014. Undifferentiated-type EGC was defined as poorly differentiated adenocarcinoma, signet ring cell carcinoma, or mucinous adenocarcinoma [5, 13]. Non-curative resection was defined as resection that failed to meet any of following criteria: en bloc resection, negative horizontal margin, negative vertical margin, tumor size ≤ 2 cm, intramucosal cancer, no ulcer, and no LVI [5, 13]. Patients with a previous history of gastric cancer, multiple gastric cancers, positive horizontal margin or piecemeal resection as the only non-curative factor, proper muscle invasion in the surgical specimen, initial follow-up loss, and additional treatment with argon plasma coagulation were excluded.
According to guidelines [5, 14], gastrectomy with LN dissection was recommended for all patients with non-curative resection. The included patients were divided into two groups: the surgery group, which comprised patients who underwent additional gastrectomy with LN dissection, and the follow-up group, which consisted of those who refused to undergo surgery because of old age, poor performance status, comorbidity, or individual preference and were followed up without further treatment.
We reviewed the medical records of enrolled patients and collected data on clinical characteristics including age, sex, American Society of Anesthesiologists (ASA) classification of physical status, and Helicobacter pylori infection and eradication status. Data on endoscopy findings, histopathology results, and surgical and follow-up outcomes were also collected. One physician for each hospital was responsible for the reliability of data collection. Collected data were independently reviewed by two authors (H.J.Y. and Y.I.K.) for validity and completeness. This study was approved by the institutional review board of each participating hospital and conducted in accordance with the principles of the Declaration of Helsinki.
ER and pathologic evaluation
ER was performed as endoscopic mucosal resection or endoscopic submucosal dissection (ESD). As described in a previous multicenter study, the typical ESD procedure was conducted in three steps: marking outside the EGC lesion, circumferential incision into the mucosa surrounding the markings for the lesion, and dissection of the submucosal layer using ESD knives [15]. During and after ESD, endoscopic hemostasis was performed using hemostatic forceps for bleeding or nonbleeding exposed vessels.
All resected specimens were stretched, formalin fixed, and cut into slices with 2-mm interval for pathological mapping, as recommended by the Japanese guidelines [13]. The horizontal and vertical resection margins, depth of tumor invasion, presence of ulcer, and LVI were evaluated along with the histological classification. Lymphatic invasion was evaluated with immunohistochemical staining using D2-40 monoclonal antibody. Vascular invasion was evaluated with hematoxylin–eosin staining. All histological subtypes were recorded in the order of the surface area occupied if there were two or more subtypes, and the tumor was classified according to its predominant subtype.
Follow-up and definitions
Patients were initially followed up with upper endoscopy 1–3 months after ER or surgical resection. They also underwent endoscopy and abdominal computed tomography with an interval of 6–12 months for 3 years and annually thereafter at least for 5 years from the initial treatment [5, 14].
Local recurrence was defined as the detection of cancer at the ER site. The follow-up duration for recurrence was defined as from the initial ER to the last available visit with surveillance examinations.
Overall survival was defined as the time from the date of the initial treatment to the date of death of any cause or the date of censoring. Medical records were reviewed with respect to the vital status of the included patients until any identifiable last date of hospital visit. In addition, data on health insurance status were also obtained, in which disqualification was considered as mortality, as suggested previously, whereas the maintenance of insurance until the date of screening (October 30, 2018) was considered as censoring [16].
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range [IQR]) and were compared using Student’s t test or Mann–Whitney U-test. Categorical variables were expressed as number (percentage) and were compared using Pearson’s Chi square test or Fisher’s exact test. Risk factors for LN metastasis in the surgical specimen were investigated using logistic regression analysis. Cumulative probabilities of developing LN or distant recurrence or the overall survival was evaluated using the Kaplan–Meier method and log-rank test. Cox regression analysis was also conducted to explore the risk factors for LN or distant recurrence during the follow-up and those for mortality. All statistical analyses were performed with SPSS (version 21.0; SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered significant.
Results
Patients
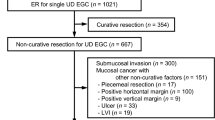
Between 2005 and 2014, a total of 1124 patients underwent ER for undifferentiated-type gastric cancer at 18 tertiary hospitals in Korea (Fig. 1). Of these, 743 (66.1%) had non-curative ER beyond the expanded criteria. A total of 109 patients were excluded from the present study for the following reasons: previous history of gastric cancer (n = 28), multiple gastric cancers (n = 16), positive horizontal margin or piecemeal resection as the only non-curative factor (n = 25), cancer invasion of the proper muscle layer in the surgical specimen (n = 6), additional treatment with argon plasma coagulation (n = 2), and initial follow-up loss (n = 32). Consequently, a total of 634 patients with non-curative ER of single undifferentiated-type EGC were included and divided into the surgery (n = 270) and follow-up (n = 364) groups.
Table 1 summarizes demographic and clinical characteristics of the included patients. The mean age ± standard deviation was 61.8 ± 11.8 years, 60.7% (385/634) were men, and 60.1% (330/549) had current H. pylori infection. Patients in the surgery group were significantly younger (p = 0.001) and more likely to have an elevated tumor (p < 0.001) and poorly differentiated adenocarcinoma (p < 0.001) than those in the follow-up group. With respect to non-curative factors, the surgery group had higher proportions of positive horizontal margin (p < 0.001), positive vertical margin (p < 0.001), submucosal invasion (p < 0.001), and LVI (p < 0.001) but a lower proportion of tumor size > 2.0 cm (p = 0.005) than the follow-up group.
Risk factors of LN metastasis at surgical resection
LN metastasis was found in 6.7% (18/270) of patients at surgical resection. In the univariate analysis, ulcer, submucosal invasion, and LVI were significantly associated with LN metastasis (all p < 0.05) (Table 2). In the multivariable logistic regression analysis with a forward variable selection procedure, ulcer (odds ratio [OR], 3.83; 95% confidence interval [CI], 1.21–12.13; p = 0.022) and submucosal invasion (OR 10.35; 95% CI 1.35–79.48; p = 0.025) were found to be independently associated with LN metastasis. When submucosal invasion was categorized into < 500 μm and ≥ 500 μm, both categories were significantly asscoatied with LN metastasis (Supplementary Table 1). However, there were no significant differences between submucosal invasion < 500 μm and ≥ 500 μm in the LN metastasis rate and in the unadjusted and adjusted ORs for LN metastasis (all p > 0.05).
Risk factors of LN or distant recurrence during the follow-up
LN or distant recurrence occurred in four patients (1.5%) during a median 58.9 (IQR 40.2–71.4) months in the surgery group and in seven (1.9%) during a median 58.1 (IQR 37.2–76.4) months in the follow-up group. In both groups, 98.3% remained without LN or distant recurrence after 5 years of follow-up (log-rank p = 0.619) (Fig. 2a).
In the surgery group, all four patients had distant metastasis: three of them died of gastric cancer but the other patient survived with systemic chemotherapy for 4 years and 4 months until the last follow-up hospital visit. Three patients had LN metastasis at the initial surgery after non-curative ER. The other patient without initial LN metastasis developed local and LN recurrence after 23 months of follow-up and, despite a second surgical resection and systemic chemotherapy, developed distant metastasis 8 months later. The initial ER finding in this patient also showed submucosal invasion with tumor size > 2.0 cm, positive horizontal and vertical margins but no ulcer and no LVI.
In the follow-up group, two patients developed local recurrence with LN metastasis after 37 and 77 months of follow-up and underwent surgical resection. The other five patients developed metastatic recurrence and died of gastric cancer. In the univariate analysis, ulcer, LVI, submucosal invasion, and positive vertical margin were associated with LN or distant recurrence (all p < 0.05) (Table 3). In the multivariable Cox regression analysis, ulcer [hazard ratio (HR) 7.60; 95% CI 1.39–35.74; p = 0.018], LVI (HR 6.80; 95% CI 1.07–42.99; p = 0.042), and positive vertical margin (HR 6.71; 95% CI 1.28–35.19; p = 0.024) were identified as independent risk factors for LN or distant recurrence.
Risk factors for mortality
During a median 84.0 (IQR 61.7–108.6) months of follow-up for overall survival, 23 and 48 patients died in the surgery and follow-up groups, respectively. The overall survival was not significantly different between the two groups (p = 0.077), although the 5- and 7-year overall survival rates were numerically higher by 5% in the surgery group (95.0% and 92.7%, respectively) than in the follow-up group (89.7% and 87.3%, respectively) (Fig. 2b).
In the multivariable Cox regression analysis, there was a significant interaction between surgery and a positive vertical margin (p = 0.004) (Supplemental Table 2). Thus, risk factors for mortality were separately analyzed in each group (Table 4). In the surgery group, only age was independently associated with mortality (HR 1.08; 95% CI 1.03–1.13; p = 0.001), whereas in the follow-up group, age (HR 1.10; 95% CI 1.06–1.14; p < 0.001), ASA class [HR (95% CI) for class II vs. I, 1.36 (0.67–2.78); for class III–IV vs. I, 4.00 (1.75–9.15); overall p = 0.002], and a positive vertical margin (HR 3.80; 95% CI 1.94–7.44; p < 0.001) were identified as independent risk factors for mortality.
Risk of LN metastasis according to number of risk factors
Because there were 20 cases of LN metastasis out of 634 patients including 18 cases in the surgery group and 2 cases in the follow-up group, we evaluated the risk of LN metastasis in the overall cohort according to the number of the risk factors for LN metastasis identified above: LVI, ulcer, submucosal invasion, and positive vertical margin. There was a positive association between the probability of LN metastasis and the number of risk factors (linear-by-linear association p < 0.001) (Table 5). The LN metastasis rates were 9.6% (14/146) in patients with two or more risk factors and 1.2% (6/488) in those with no or one risk factor.
Discussion
In this nationwide multicenter cohort study involving > 600 patients, which is the largest cohort thus far of patients with undifferentiated-type EGC with non-curative ER, we showed that LVI, ulcer, submucosal invasion, and positive vertical margin were associated with an increased risk of LN or distant metastasis and mortality. Ulcer and submucosal invasion were independently associated with LN metastasis in the surgery group. Ulcer, LVI, and positive vertical margin were associated with LN or distant recurrence in the follow-up group. In addition, positive vertical margin was associated with increased mortality in the follow-up group but not in the surgery group. Furthermore, the risk of LN metastasis was considerably high in patients with two or more of the above-mentioned risk factors. Our results indicate that patients with two or more risk factors would substantially benefit from surgical resection after non-curative ER of undifferentiated-type EGC.
Previous cohort studies that evaluated the risk factors of LN metastasis after non-curative ER mainly included differentiated-type EGCs [6,7,8,9,10]. A recently reported Japanese multicenter study included the largest number (n = 1969) of patients with non-curative ER; however, there were only 292 patients with undifferentiated-type EGCs [8]. More importantly, the potential risk factors of LN metastasis were categorized for differentiated-type cancer (tumor size > 3 cm and submucosal invasion ≥ 500 μm from the muscularis mucosa), which differed from those for undifferentiated-type cancer (tumor size > 2 cm and submucosal invasion including < 500 μm). This might have introduced bias in the results for undifferentiated-type cancers. In the present study, we included undifferentiated-type EGCs only and used categories suitable for undifferentiated-type cancers. Therefore, we could evaluate the risk factors for LN or distant metastasis specifically for undifferentiated-type EGC.
In our study, submucosal invasion and ulcer were associated with LN metastasis in the surgery group, and ulcer and LVI were associated with LN or distant recurrence in the follow-up group. These findings are consistent with those of previous surgical studies showing that these factors were associated with LN metastasis in undifferentiated-type cancers [11, 17,18,19,20,21,22]. However, prior studies on non-curative ER have not suggested submucosal invasion < 500 μm or ulcer as risk factors for LN metastasis. Only LVI was highlighted as the risk factor of LN metastasis at surgery [9], of LN or distant recurrence during the follow-up [8, 10], and of mortality during the follow-up without surgery [12]. Several other studies suggested submucosal invasion ≥ 500 μm or submucosal invasion with a positive vertical margin, as well as LVI or venous invasion, as risk factors [7, 10]. This discrepancy may be because the majority of patients with non-curative ER had differentiated-type cancers, in whom the risk of LN metastasis is still minimal if the tumor size is ≤ 3 cm despite having submucosal invasion < 500 μm or ulcer. However, several detailed investigations using surgical data showed that the risk of LN metastasis increased even with submucosal invasion < 500 μm compared with mucosal invasion in undifferentiated-type cancer [17, 18, 23]. In our study, the effect size for submucosal invasion in the surgery group and ulcer in the surgery and follow-up groups was similar to that of LVI in the follow-up group. Therefore, our results suggest that submucosal invasion and ulcer should be considered important as LVI with respect to the risk of LN metastasis after non-curative ER of undifferentiated-type EGC.
A positive vertical margin was a risk factor for LN or distant recurrence and mortality in the follow-up group. Importantly, the effect of a positive vertical margin on mortality was significantly affected by surgical resection, and a positive vertical margin was not associated with mortality in the surgery group. In our study, we excluded six patients who had proper muscle invasion at surgery because they had advanced gastric cancer. When we conducted the analysis without excluding them, positive vertical margin was found to be an independent risk factor for LN metastasis also in the surgery group (data not shown). However, this exclusion was not possible in the follow-up group because the depth of invasion could not be accurately evaluated. Consequently, a few patients with advanced gastric cancer might have been included in the follow-up group. Therefore, surgical resection should be recommended for non-curative ER with a positive vertical margin because of not only possible LN metastasis, but also potential residual cancer.
To identify patients for whom additional surgery can be strongly recommended, the risk of LN metastasis were further stratified according to the number of identified risk factors. We could show that the risk of LN metastasis in patients who had two or more risk factors among LVI, ulcer, submucosal invasion and positive vertical margin was 9.6% while the risk was only 1.2% in patients with no or one risk factor. The risk factors differed among for LN metastasis in the surgery group, for LN or distant recurrence in the follow-up group, and for mortality. This discrepancy might have been due to the difference in the clinical characteristics of the patients included in each group and the difference in the outcome variables. However, the risk stratification based on these findings provided simple prediction that can be easily applied in the clinical practice. In our analysis, age and ASA class were also independent predictive factors of survival in the follow-up group. It was previously shown that underlying disease independently affected the overall survival of patients who were followed up without surgery after non-curative ER of differentiated-type EGC [12]. Thus, a simple follow-up may be recommended for elderly patients with ASA class III-IV if they have no or one risk factor because they would not gain a large benefit from surgical treatment. However, if they have two or more risk factors, surgery may be carefully considered.
The strength of our study was the use of nationwide large-scale data that represent patients with undifferentiated-type EGC in Korea. The long-term follow-up duration (median 5 years of surveillance and 7 years of survival) allowed us to detect multiple risk factors for LN or distant recurrence during the follow-up and those for mortality despite small numbers of these events. The utilization of health insurance status data was particularly important because patients who refused surgery because of old age or underlying illness were also less likely to undergo surveillance examinations. This enabled us to uncover the importance of a positive vertical margin for survival. The detailed manual review of medical records by physicians and independent validation of collected data may have further strengthened the validity of our results. However, several limitations also need to be mentioned. First, the retrospective design and consequent lack of histopathology review, including the lack of information on mixed histology, precluded further elucidation of potential risk factors for LN metastasis or mortality. Second, the retrospective nature of our study might also have induced selection biases during treatment selection between ER and surgery before ER and between additional surgery and follow-up after non-curative ER. Further prospective cohort study with pre-specified protocol can reduce this bias. Third, the curative ER rate was as low as 33.9% (381/1124) in our study. This was because some cases were not diagnosed as undifferentiated-type EGC before ER or were treated with ER even they were out-of-indication in the evaluation before ER. In addition, preoperative diagnosis for tumor size or depth might have been inaccurate in our study. Nevertheless, this low rate of curative ER might not have affected our results because our findings were based on the final pathology results after ER. Fourth, although the sample size was large, the study power was not sufficient because the number of LN or distant metastasis and mortality were small, which might have led to a type II error.
In conclusion, in this nationwide multicenter cohort study, we found that LVI, ulcer, submucosal invasion, and positive vertical margin were independently associated with LN or distant metastasis after non-curative ER of undifferentiated-type EGC. The patients with two or more risk factors had high risk of LN metastasis. Our results emphasize the importance of a detailed pathological assessment of these non-curative factors from the ER specimen. Our study suggests that surgical resection should be strongly recommended for patients with undifferentiated-type EGC if they have two or more of the identified risk factors after non-curative ER.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Kim SG, Seo HS, Lee HH, Song KY, Park CH. Comparison of the Differences in Survival Rates between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5,507 Patients in Korea. J Gastric Cancer. 2017;17:212–9.
Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490–9.
Guideline Committee of the Korean Gastric Cancer Association, Development Working Group, Review Panel. Korean Practice Guideline for Gastric Cancer. an Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer. 2018;2019(19):1–48.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01042-y.
Kim ER, Lee H, Min BH, Lee JH, Rhee PL, Kim JJ, et al. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2015;102:1394–401.
Yang HJ, Kim SG, Lim JH, Choi J, Im JP, Kim JS, et al. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg Endosc. 2015;29:1145–55.
Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175–84.
Kawata N, Kakushima N, Takizawa K, Tanaka M, Makuuchi R, Tokunaga M, et al. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017;31:1607–16.
Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679–89.
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–52.
Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, et al. Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy. 2012;44:1114–20.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104.
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–35.
Kim JH, Kim K, Park SJ, Lee JY, Kim K, Lim MC, et al. Comparative Effectiveness Of Abdominal Versus Laparoscopic Radical Hysterectomy for cervical cancer in the Postdissemination Era. Cancer Res Treat. 2019;51:788–96.
Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498–503.
Ye BD, Kim SG, Lee JY, Kim JS, Yang HK, Kim WH, et al. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undifferentiated early gastric cancer. J Gastroenterol Hepatol. 2008;23:46–50.
Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, et al. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008;15:764–9.
Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10.
Chung JW, Jung HY, Choi KD, Song HJ, Lee GH, Jang SJ, et al. Extended indication of endoscopic resection for mucosal early gastric cancer: analysis of a single center experience. J Gastroenterol Hepatol. 2011;26:884–7.
Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, et al. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg. 2012;99:1688–92.
Lee IS, Lee S, Park YS, Gong CS, Yook JH, Kim BS. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg Oncol. 2017;26:8–12.
Acknowledgements
The authors also acknowledge the valuable contribution of Boram Park, PhD, Biostatistics Collaboration Team, Research Core Center, Research Institute, National Cancer Center, Goyang, Korea, who provided statistical analyses of data.
Funding
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research (Woon Geon Shin 2017 and Hyo-Joon Yang 2018) and by the National Research Foundation of Korea (Grant No. 2020R1G1A1010927).
Author information
Authors and Affiliations
Contributions
H-JY contributed to the design of the study; collection, validation, analysis, and interpretation of data; and drafting of the manuscript. J-YJ contributed to collection of data and critical revision of the manuscript for important intellectual content. SGK contributed to collection of data and critical revision of the manuscript for important intellectual content. JYA contributed to the conception of the study, collection of data, and critical revision of the manuscript for important intellectual content. SYN contributed to collection of data and critical revision of the manuscript for important intellectual content. J-HK contributed to the conception of the study, collection of data, and critical revision of the manuscript for important intellectual content. B-HM contributed to collection of data and critical revision of the manuscript for important intellectual content. WSL contributed to collection of data and critical revision of the manuscript for important intellectual content. BEL contributed to collection of data and critical revision of the manuscript for important intellectual content. MKJ contributed to collection of data and critical revision of the manuscript for important intellectual content. JMP contributed to collection of data and critical revision of the manuscript for important intellectual content. WGS contributed to the conception of the study, collection of data, and critical revision of the manuscript for important intellectual content. HLL contributed to collection of data and critical revision of the manuscript for important intellectual content. T-GG contributed to collection of data and critical revision of the manuscript for important intellectual content. MIP contributed to collection of data and critical revision of the manuscript for important intellectual content. JC contributed to collection of data and critical revision of the manuscript for important intellectual content. CH Tae contributed to collection of data and critical revision of the manuscript for important intellectual content. Y-IK contributed to collection and validation of data and critical revision of the manuscript for important intellectual content. IJC contributed to the conception of the study, collection of data, and critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, HJ., Jang, JY., Kim, S.G. et al. Risk factors of lymph node metastasis after non-curative endoscopic resection of undifferentiated-type early gastric cancer. Gastric Cancer 24, 168–178 (2021). https://doi.org/10.1007/s10120-020-01103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01103-2