Abstract
Pyloric gland adenoma (PGA) is an uncommon variant of gastric adenoma exhibiting pyloric gland/mucous neck cell differentiation. We present a sporadic PGA associated with a large fundic gland polyp (FGP) in a woman in her 40 s without Helicobacter pylori infection. The polyp, measuring 25 mm in size, was located in the middle gastric body and was removed by endoscopic submucosal dissection. Histological examination revealed three morphologically distinct components: FGP, FGP with large cysts, and PGA. A genetic analysis identified a truncating APC mutation in all the three components, supporting their histogenetic relationship. Additionally, a GNAS mutation was detected in two components, FGP with large cysts and PGA, whereas a KRAS mutation was exclusively found in the PGA component. Thus, despite the unusual presentation, the PGA component harbored prototypical genetic alterations. The differential genetic alterations observed in the three components imply that they represent stepwise progression from FGP to PGA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyloric gland adenoma (PGA) is an increasingly recognized variant of adenoma, most commonly located in the stomach and duodenum [1]. Gastric PGAs either occur sporadically or in patients with familial adenomatous polyposis (FAP) [2]. Sporadic gastric PGAs usually develop on a background of metaplastic mucosa due to Helicobacter pylori (H. pylori) infection or autoimmune gastritis [1, 2]. Despite its name, PGAs exhibit immunohistochemical profiles similar to mucous neck cells of fundic glands rather than pyloric glands, as indicated by the weak expression of pepsinogen-I in addition to diffuse MUC6 expression [3]. Consistently, PGAs are primarily located in the gastric body, which is normally covered by the fundic gland mucosa. Accordingly, it is suggested that pseudopyloric metaplasia occurring in the fundic gland mucosa, which is induced by sustained inflammation, plays a critical role in the development of sporadic PGAs [3].
In contrast, FAP-associated PGAs are observed on the pristine fundic gland mucosa, often in association with fundic gland polyposis [4]. Fundic gland polyps (FGPs) in patients with FAP have clinicopathological features distinct from sporadic lesions [2]. They occur in younger patients with an almost equal gender distribution and commonly present as polyposis. Foveolar dysplasia is frequent and seen in up to 41% of FAP-associated FGPs [5, 6]. In contrast to the innocuous nature of sporadic FGPs, FAP-associated FGPs are thought to have risks for neoplastic progression and may be precursors of foveolar-type adenoma and PGA, which are detected with an increased prevalence in FAP patients [4].
Based on the highly different backgrounds of sporadic and FAP-associated PGAs, it is expected that their histogenetic processes are distinct. However, the PGAs occurring in these two different situations are similar not only morphologically, but also genetically. Our previous studies showed the common presence of GNAS, KRAS, and APC mutations in both sporadic and FAP-associated PGAs [7, 8]. Notably, GNAS mutations are virtually absent and KRAS mutations are rare among adenomas of other histological subtypes and adenocarcinomas of the stomach [7]; thus, the mutation profile of PGA is quite distinct among gastric tumors.
Here, we present an unusual case of PGA associated with a large sporadic FGP. The endoscopic observation successfully detected the presence of the PGA component and our genetic analysis revealed the stepwise progression from FGP to PGA in this unique lesion.
Case report
A female patient in her 40 s underwent esophagogastroduodenoscopy for sustained nausea and epigastric pain at a local hospital, and was found to have a large gastric polyp. The patient had been treated with a proton pump inhibitor (Vonoprazan) for 2 months before being referred to our hospital. She previously had a radical hysterectomy and chemotherapy for immature teratoma at the age of 27 years and there have been no signs of recurrence during the 10-year follow-up at our institution. This patient had not received colonoscopic examination, but had no past or family history suggestive of FAP.
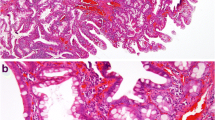
Endoscopic examination revealed a pedunculated polyp with uneven surface, measuring 25 mm in size, on the greater curvature of the middle gastric body (Fig. 1a). A well-demarcated whitish area was recognized on the distal side of the polyp (Fig. 1b). Additionally, a superficial elevated component was located on the periphery of the polyp (Fig. 1c). On magnifying narrow-band imaging (M-NBI), the whitish area showed a mildly irregular microsurface pattern (Fig. 1d). Most of the pedunculated component of the polyp showed elongated pits with expanded intervening part (Fig. 1e). The superficial elevated component showed slightly elongated pits (Fig. 1f). The background gastric mucosa showed no atrophic change and the urea breath test was negative, indicating the absence of H. pylori infection. In addition, the patient had not received H. pylori eradication therapy previously.
Endoscopic images. a White light imaging (WLI) in a forward view. A large solitary polyp with uneven surface was located on the greater curvature of the gastric body. The background mucosa was non-atrophic. b Underwater observation in a retroflexed view showing a semi-pedunculated morphology. A well-demarcated whitish area (arrowheads) was observed on the distal side of the polyp. c A superficial elevated component was recognized on the periphery of the polyp (arrows). The whitish area detected in the underwater observation is indicated by arrowheads. This image was taken after placing cautery marks for endoscopic submucosal dissection. d-f Magnifying narrow-band imaging with the water-immersion technique. A mildly irregular microsurface pattern in the whitish area recognized in WLI (d). Elongated microsurface pattern with the expansion of the intervening part is seen on the top of the polyp (e). The superficial elevated component shows slightly enlarged pits (f)
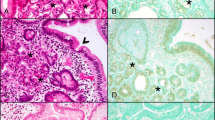
Biopsy specimens obtained from the polyp showed FGP with dysplasia and another specimen taken from the whitish area showed PGA. Based on the biopsy diagnosis, we performed endoscopic submucosal dissection and achieved en bloc resection. Histological examination revealed the presence of three distinct components in the polyp: (1) FGP, (2) FGP with large cysts, and (3) PGA (Fig. 2). The FGP component was observed on the periphery of the lesion and exhibited low-grade foveolar dysplasia (Fig. 3a, b). The FGP with large cysts constituted the largest component of the polyp. This component also exhibited histological features of FGP, but the cysts were markedly dilated, measuring up to 1500 μm in diameter (Fig. 3c-e). The overlying foveolar epithelium of this component also showed low-grade dysplasia. The whitish area recognized in the endoscopic examination was PGA, which was in agreement with the biopsy diagnosis (Fig. 3f, g). Immunohistochemically, the PGA component showed diffuse expression of MUC6 except for the superficial epithelium, which was positive for MUC5AC (Fig. 3h, i). The surrounding mucosa was fundic gland mucosa without atrophy, metaplasia, or inflammation.
Histologically distinct components of the polyp. a Panoramic view of the polyp. Note the prominently dilated cysts occupying a large proportion of the polyp. b The polyp consisted of three histologically distinct components. Fundic gland polyp (FGP) with large cysts constituted the large proportion of the polyp. Pyloric gland adenoma (PGA) component was located in the whitish area recognized in the endoscopic examination. FGP component, recognized as a superficial elevated component, was observed on the periphery of the polyp. c Arrows indicate the FGP component exhibiting less prominent cystic changes compared with the other part of the polyp
Histological features of the polyp. a, b FGP with foveolar dysplasia on the periphery of the polyp. This component showed cystic changes of the fundic glands. Some of the glands showed irregular contours (a). The overlying epithelium exhibited low-grade dysplasia (b). c-e FGP with large cysts. The cysts were markedly dilated, measuring up to 1500 μm in diameter (c). The cysts were lined by oxyntic epithelium (d), or less frequently, foveolar epithelium (e). f-i Pyloric gland adenoma (PGA) component consisting of proliferation of tubules lined by mucous cells with characteristic ground-glass cytoplasm (f, g). The PGA component showed diffuse MUC6 expression (h) with a surface coating of MUC5AC (i)
To elucidate the genetic characteristics of this lesion, we analyzed the respective components for APC, KRAS, and GNAS mutations by Sanger sequencing as described previously (Fig. 4) [8]. The analysis identified a truncating APC mutation in all the three components of the polyp. A GNAS mutation was also detected in the areas of FGP with large cysts and PGA. A KRAS mutation was exclusively present in the PGA component.
Genetic alterations in the respective components. a An identical APC mutation was detected in all three components, but not in the normal mucosa. A GNAS mutation was additionally detected in the FGP with large cysts and PGA. A KRAS mutation was exclusive to the PGA component. b A putative genetic progression model of the lesion
Discussion
The present case showed a unique association of three histologically distinct components: FGP, FGP with large cysts, and PGA, with the FGP with markedly dilated cysts constituting the largest component of the polyp. FGPs usually present as small polyps; however, there have been a few reports of solitary large FGPs up to 8 cm [9, 10]. Although detailed histological findings of those lesions were not available, at least one of the lesions showed foveolar dysplasia, similar to that observed in our case.
The presence of the PGA component was endoscopically detectable as a demarcated whitish area. M-NBI further highlighted the PGA component as an area of a distinct microsurface pattern. Although the significance of the present M-NBI findings in the diagnosis of PGA remains elusive, our endoscopic examination and biopsy successfully made the diagnosis of the polyp prior to its removal.
The three histologically distinct components exhibited different APC, GNAS, and KRAS mutation statuses. The shared presence of an identical APC mutation, along with their topographical association, indicates that all the three components are histogenetically related. The additional presence of the GNAS mutation in the areas of FGP with large cysts and PGA as well as the KRAS mutation in the PGA component imply that the three components represent the process of stepwise progression to PGA (Fig. 3b).
The vast majority of sporadic FGPs harbor activating CTNNB1 mutations whereas FAP-associated and a minor subset of sporadic FGPs have truncating APC mutations [11,12,13]. Although both of these mutations play oncogenic roles through stabilization of β-catenin, these genetic alterations are of somewhat different biological significance. Sporadic FGPs are generally non-dysplastic and have negligible risks of malignant progression. In contrast, FAP-associated and sporadic FGPs with APC mutations often develop foveolar dysplasia, as seen in the present case [5, 10, 11, 14].
Although cystic change is one of the histological hallmarks of FGPs, the remarkably dilated cysts seen in the large proportion of the polyp are an unusual finding, even for FGPs. A recent study described that FGPs in patients treated with proton pump inhibitors exhibit enlarged cyst formation. However, the maximum cyst size of the respective FGPs in patients treated by proton pump inhibitors was reported to be 387 μm on average [15], which is still considerably smaller than the cysts observed in the present case. Our genetic analysis identified a GNAS mutation, in addition to an APC mutation, in the area with markedly dilated cysts. GNAS mutations constitutively activate the protein kinase A pathway, which promotes electrolyte, water, and mucin secretion in epithelial cells [16, 17]. We expect that the markedly dilated cyst formation in this component is the consequence of enhanced secretory functions induced by the GNAS mutation.
The present case is an unusual example of PGA because of its occurrence on the background of pristine mucosa and association with an FGP component. However, despite the unique presentation, the PGA component showed the classical immunohistochemical phenotype and genetic alterations, which are common to both sporadic and FAP-associated lesions. It has been suggested that FAP-associated PGAs may arise from FGPs; however, the direct demonstration of their histogenetic relationship is practically difficult because of the multiplicity of FAP-associated FGPs. Our case, presenting as a solitary polyp on a background of non-atrophic fundic gland mucosa, indicates that PGAs can arise from APC-mutated FGPs.
References
Vieth M, Kushima R, Borchard F, et al. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch. 2003;442:317–21.
Abraham SC. Fundic gland polyps: common and occasionally problematic lesions. Gastroenterol Hepatol (NY). 2010;6:48–51.
Kushima R, Sekine S, Matsubara A, et al. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318–25.
Wood LD, Salaria SN, Cruise MW, et al. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38:389–93.
Wu TT, Kornacki S, Rashid A, et al. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998;22:293–8.
Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180–5.
Matsubara A, Sekine S, Kushima R, et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2012;229:579–87.
Hashimoto T, Ogawa R, Matsubara A, et al. Familial adenomatous polyposis-associated and sporadic pyloric gland adenomas of the upper gastrointestinal tract share common genetic features. Histopathology. 2015;67:689–98.
Winkler A, Hinterleitner TA, Langner C. Giant fundic gland polyp mimicking a gastric malignancy. Endoscopy. 2007;39(Suppl 1):E34.
El Hajj I, Hawchar M, Soweid A, et al. Giant sporadic fundic gland polyp: endoscopic and endosonographic features and management. World J Gastroenterol. 2008;14:6593–5.
Abraham SC, Nobukawa B, Giardiello FM, et al. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol. 2000;157:747–54.
Abraham SC, Nobukawa B, Giardiello FM, et al. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005–100.
Sekine S, Shibata T, Yamauchi Y, et al. Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch. 2002;440:381–6.
Abraham SC, Park SJ, Mugartegui L, et al. Sporadic fundic gland polyps with epithelial dysplasia: evidence for preferential targeting for mutations in the adenomatous polyposis coli gene. Am J Pathol. 2002;161:1735–42.
Fukuda M, Ishigaki H, Sugimoto M, et al. Histological analysis of fundic gland polyps secondary to PPI therapy. Histopathology. 2019;75:537–45.
Makita N, Sato J, Rondard P, et al. Human G(salpha) mutant causes pseudohypoparathyroidism type Ia/neonatal diarrhea, a potential cell-specific role of the palmitoylation cycle. Proc Natl Acad Sci USA. 2007;104:17424–9.
Nishikawa G, Sekine S, Ogawa R, et al. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer. 2013;108:951–8.
Acknowledgements
We thank Ms. Reiko Ogawa, Ms. Sachiko Miura, and Ms. Toshiko Sakaguchi for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nonaka, S., Hashimoto, T., Oda, I. et al. Sporadic pyloric gland adenoma associated with a large fundic gland polyp: genetic evidence for stepwise progression. Gastric Cancer 23, 1102–1106 (2020). https://doi.org/10.1007/s10120-020-01082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01082-4