Abstract
Background
The incidence and clinical presentation of internal hernia after gastrectomy have been changing in the minimally invasive surgery era. This study aimed to analyze the clinical features and risk factors for internal hernia after gastrectomy for gastric cancer.
Methods
We retrospectively analyzed internal hernia after gastrectomy for gastric cancer in 6474 patients between January 2003 and December 2016 at Seoul National University Bundang Hospital. Multivariable logistic regression was performed to evaluate risk factors.
Results
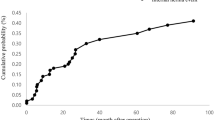
Internal hernias identified by computed tomography or surgical exploration were 111/6474 (1.7%) and the median interval time was 450 days after gastrectomy. Fourteen (0.9%) of the 1510 patients who underwent open gastrectomy and 97 (2.0%) of the 4964 patients who underwent laparoscopic gastrectomy developed internal hernia. Of the 6474 patients, internal hernia developed in 0 (0%), 9 (1.1%), 40 (3.1%), 56 (3.3%), 6 (2.3%), and 0 (0%) patients who underwent Billroth I, Billroth II, Roux-en-Y, uncut Roux-en-Y, double tract, and esophagogastrostomy reconstructions, respectively. Fifty-nine (53.2%) of 111 patients with symptomatic hernia underwent surgery. Of the 59 internal hernias, treated surgically, 32 (53.2%), 27 (45.8%), and 0 (0%) were identified in jejunojejunostomy mesenteric, Petersen’s, and transverse colon mesenteric defects, respectively. In multivariate analysis, non-closure of mesenteric defects (P < 0.01), laparoscopic approach (P < 0.01), and totally laparoscopic approach (P = 0.03) were independent risk factors for internal hernia.
Conclusions
The potential spaces such as Petersen’s, jejunojejunostomy mesenteric, and transverse colon mesenteric defects should be closed to prevent internal hernia after gastrectomy for gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic gastrectomy has been widely adopted as a treatment for gastric cancer, not only in Eastern Asia but also in Western countries. It is minimally invasive and reduces the risk of postoperative adhesions; thus, enabling earlier recovery of bowel movement and reduced hospital stay. Moreover, several trials have reported that the long-term oncologic outcome of laparoscopic surgery is similar to that of conventional open surgery [1,2,3,4,5,6].
Gastric cancer surgery includes lymph-node dissection and bowel reconstruction for bowel continuity after gastrectomy. During this process, internal hernia could develop. The incidences of internal hernia were reported from less than 1–5% of patients after open gastrectomy [7,8,9]. After the adoption of laparoscopic surgery, the incidences have increased to 5–9% [10,11,12,13,14]. Delay in management for internal hernia might result in intestinal strangulation and the subsequent need for bowel resection [4, 14, 15].
Although several studies have reported various complications after gastrectomy for gastric cancer, only a few studies have evaluated late postoperative complications, especially internal hernia [3, 6, 16]. The aim of this study was to analyze the clinical characteristics of internal hernia after introduction of minimally invasive surgeries and to evaluate the risk factors of internal hernia to prevent the life-threatening consequences.
Materials and methods
From January 2003 to December 2016, 7041 patients underwent gastrectomy for gastric cancer at Seoul National University Bundang Hospital, Seongnam, South Korea. Of these, 567 palliative gastrectomies were excluded because information regarding long-term complications including internal hernia could not be gathered for these patients. Consequently, 6474 patients who underwent curative gastrectomy were retrospectively enrolled in this study.
The study retrospectively analyzed patients with internal hernia identified by surgical exploration or abdominal computed tomography (CT) from surgical and medical records. The median follow-up was 60.3 months after gastrectomy.
The procedures related to internal hernia were distal gastrectomy with Billroth II, Roux-en-Y and uncut Roux-en-Y reconstructions, total gastrectomy with Roux-en-Y reconstruction, and proximal gastrectomy with double tract reconstruction. Internal hernia was diagnosed through surgical exploration or CT, which showed whirling appearance of the mesentery and mesenteric vessels (whirl sign). Patients’ age, sex, body mass index (BMI), history of previous abdominal surgery, mesenteric defect closure (no-closure versus closure), operative approach (open versus laparoscopic), number of ports (multi-port versus single port), type of gastrectomy, type of reconstruction, and route of reconstruction were evaluated for internal hernia.
Categorical data were compared using Pearson’s Chi-squared test, and continuous data were compared using the independent t test. Categorical variables were presented as numbers with percentages, and continuous data were expressed as mean ± standard deviation. The risk factors for internal hernia were evaluated using logistic regression analysis. Analyses were conducted using IBM SPSS Statistics version 21 (IBM Co., Armonk, NY, USA) statistical software. A P value, less than 0.05 was considered statistically significant.
Results
Of the 6474 patients, 111 (1.7%) developed internal hernia. The median time to diagnosis of internal hernia was 450 days (3–3398 days).
The characteristics of patients with internal hernia are shown in Table 1. Internal hernia developed in 82 (1.9%) of the 4333 male patients and in 29 (1.4%) of the 2141 female patients (P = 0.1).
The mean BMI at the time of gastrectomy and at the diagnosis of internal hernia was 23.5 ± 3.3 kg/m2 and 20.4 ± 2.7 kg/m2, respectively. The change of the BMI was that 106 (95.5%) of the 111 patients showed decreased BMI and 5 (4.5%) showed increased BMI. The ratios for BMI decrease and increase were 11.7% ± 5.9% and 3.5% ± 3.7%, respectively.
Sixteen (1.4%) of the 1177 patients who had a history of previous abdominal surgery except gastrectomy developed internal hernia, compared to 95 (1.8%) of the 5297 patients without a history of previous abdominal surgery (P = 0.3).
In our center, we have standardly closed the mesenteric defects from 2015. Eighty-eight (3.1%) of the 2826 patients without closed defects and 23 (1.8%) of the 1248 patients with closed defects developed internal hernia; the incidence of internal hernia was significantly higher in the non-closure group (P = 0.02).
The incidence of internal hernia according to gastrectomy type is shown in Table 2. Fourteen (0.9%) of the 1510 patients who underwent open gastrectomy and 97 (2.0%) of the 4964 patients who underwent laparoscopic gastrectomy developed internal hernia (P < 0.01).
Eighty-three (1.8%) of 4653 patients who underwent a multi-port approach and 14 (4.5%) of 311 patients who underwent a single-port approach developed internal hernia (P < 0.01).
Totally laparoscopic surgery has been started from 2012 in our center. As getting accustomed to laparoscopic surgery, we started performing this approach because of its less postoperative pain and better cosmetic outcomes than the laparoscopy-assisted approach. Internal hernia developed in 56 (1.9%) of the 3010 patients who underwent laparoscopy-assisted gastrectomy, and 41 (2.1%) of the 1954 patients who underwent totally laparoscopic gastrectomy (P = 0.55).
In terms of gastrectomy type, internal hernia developed in 72 (2.7%) of the 2688 patients who underwent distal gastrectomy, in 33 (2.9%) of the 1122 patients who underwent total gastrectomy, and in 6 (2.3%) of the 264 patients who underwent proximal gastrectomy (P = 0.81).
Regarding the reconstruction type, internal hernia developed in 9 (1.1%) of the 832 patients with Billroth II reconstruction, in 40 (3.1%) of the 1272 patients with Roux-en-Y reconstruction, in 56 (3.3%) of the 1706 patients with uncut Roux-en-Y reconstruction, and in 6 (2.3%) of the 264 patients with double tract reconstruction. The incidence of internal hernia was significantly higher after uncut Roux-en-Y and Roux-en-Y reconstruction than after Billroth II reconstruction (P < 0.01 and P < 0.01).
Regarding the route of reconstruction, internal hernia developed in 110 (2.7%) of the 4038 patients with antecolic route, and in 1 (1.7%) of the 60 patients with retrocolic route (P = 0.62).
The sites and treatments of internal hernia are shown in Table 3. Fifty-nine (53.2%) of the 111 patients underwent surgical treatments; of these, 33 (55.9%) patients underwent the laparoscopic approach. Of the 59 surgically treated patients with internal hernia, 32 (54.2%) were located at the jejunojejunostomy mesenteric defect and 27 (45.8%) were found at the Petersen’s defect, which is the space between the Roux limb and the transverse mesocolon. There was no internal hernia at transverse colon mesenteric defect among the surgically treated patients. Two (1.8%) patients died due to septic shock caused by bowel strangulation. Nine (8.1%) patients had Clavien–Dindo grade III surgical complications or higher.
Univariate analysis showed that the non-closure of mesenteric defects [odds ratio (OR) 1.72, 95% CI 1.09–2.78, P = 0.02] and the laparoscopic approach (OR 2.27, 95% CI 1.27–4.07, P = 0.01) were significant risk factors for internal hernia. Multivariate analysis demonstrated that non-closure of mesenteric defects (OR 2.08, 95% CI 1.29–3.33, P < 0.01), laparoscopic approach (OR 2.43, 95% CI 1.35–4.38, P < 0.01), and totally laparoscopic approach (OR 1.65, 95% CI 1.05–2.61, P = 0.03) were independent risk factors for internal hernia (Table 4).
Discussion
In this study, we investigated the clinical features and risk factors of internal hernia in patients who underwent gastrectomy for gastric cancer. The closure of Petersen’s, jejunojejunostomy mesenteric and transverse colon mesenteric defects is important for the prevention of internal hernia after gastrectomy.
Recently, laparoscopic surgery has been increasingly performed because of its many advantages compared to open surgery, such as reduced perioperative morbidity and a shortened hospital stay. Several studies on laparoscopic gastrectomy have reported additional benefits, including a better quality of life and an oncologic outcome comparable to open gastrectomy [1,2,3,4,5,6].
Internal hernia is a critical problem after laparoscopic and open surgery because they might cause life-threatening conditions, such as bowel strangulation or perforation [17, 18]. Furthermore, the incidence of internal hernia has increased to 5–9% after the adoption of laparoscopic surgery [10,11,12,13,14]. Therefore, the evaluation of risk factors is important to prevent this complication. However, there is limited information on internal hernia after gastric cancer surgery.
The present study showed that the overall incidence of internal hernia was 1.7%. Other reported incidences of internal hernia after gastric cancer surgery range from less than 1–7% [4, 14, 15, 19].
Excessive weight loss might be one of the risk factors for internal hernia after gastrectomy. In our study, BMI decreased in 106 (95.5%) of the 111 patients with internal hernia between the time of gastrectomy and the time of diagnosis of internal hernia. The mean BMI reduction rate of these patients was 11.7, which was larger than that reported in other studies (range 6.6–10.8%) [20, 21]. Excessive weight loss is considered one of the risk factors for internal hernia in bariatric surgery [22, 23]. It is presumed that stitches closing the mesentery defects may loosen as the amount of mesenteric fat tissue decreases due to excess body weight loss. This may contribute to the development of internal hernias even after closure of the defects [4, 24, 25].
From studies of gastric cancer surgery, the closure of the mesenteric defects is recommended to reduce the incidence of internal hernia [14, 15, 19]. In this study, the closure of these defects was associated with a significantly lower incidence of internal hernia compared to the non-closure of the defects, and the non-closure of the defects was demonstrated as a risk factor for internal hernia by multivariate analysis. Paroz et al. [7] suggested the closure of all mesenteric defects using a continuous nonabsorbable suture to prevent internal hernia after laparoscopic Roux-en Y gastric bypass (internal hernia incidence: 5.6% absorbable versus 1.3% nonabsorbable; P = 0.03).
Several studies have reported that the incidence of postoperative internal hernia was significantly higher after laparoscopic gastrectomy for gastric cancer than after open gastrectomy. Laparoscopy might cause less trauma, leading to fewer adhesions. Hence, the intestine is less adhered to adjacent structures, which may lead to better mobility, thereby enabling it to move into the mesenteric defects [14, 15, 19]. In our study, the incidence of internal hernia was significantly higher in laparoscopic surgery than in open surgery, and the laparoscopic approach was demonstrated as a risk factor for internal hernia by multivariate analysis.
The present study showed higher incidence of internal hernia after single-port surgery compared to multi-port surgery. There were no studies comparing the incidence of internal hernia in regard to the number of ports. In our experience, the outcome might have been attributed to the relatively difficult manipulation of laparoscopic instruments in single-port surgeries. For a free range of motion and reducing the clash between instruments, optimizing the distance between ports is essential, which cannot be fulfilled in single-port surgery [26]. Therefore, meticulous suture to seal the mesenteric defects becomes more demanding for single-port surgery. However, the number of ports was not an independent risk factor for internal hernia by multivariate analysis.
Totally laparoscopic gastrectomy has a higher rate of postoperative internal hernia than laparoscopy-assisted gastrectomy because it is associated with fewer adhesions. In laparoscopy-assisted surgery, a surgeon directly manipulates the bowel through the mini-laparotomy site, causing more adhesions than in totally laparoscopic surgery [19]. Multivariate analysis in this study showed a significantly higher risk for internal hernia in totally laparoscopic surgery than in laparoscopy-assisted surgery.
Kelly et al. [14] reported that internal hernia rarely developed in patients who underwent Billroth II reconstruction than in those who underwent Roux-en Y reconstruction. In the present study, Roux-en Y and uncut Roux-en Y reconstructions showed a significantly higher incidence of internal hernia than Billroth II reconstruction. It might be because Roux-en Y and uncut Roux-en Y reconstruction types have two defects (jejunojejunostomy mesenteric defect and Petersen’s defect) for internal hernia, whereas Billroth II reconstruction has only a Petersen’s defect. However, the reconstruction type was not an independent risk factor for internal hernia by multivariate analysis.
The retrocolic route has three potential spaces for internal hernia, the jejunojejunostomy mesenteric, the transverse colon mesenteric, and Petersen’s defect. For this reason, many surgeons have preferred the antecolic route, which has only two potential spaces, the jejunojejunostomy mesenteric and Petersen’s defect, to reduce the incidence of internal hernia [13, 18, 27, 28]. However, there was not significant difference of the incidence of internal hernia between two groups, and the type of reconstruction route was not an independent risk factor for internal hernia by multivariate analysis.
In this study, 52 (46.8%) of the 111 patients underwent non-surgical treatment for internal hernia. Indication for non-surgical treatment for internal hernia has not been clearly established yet. Lannelli et al. [29] recommended to consider early surgical exploration if there is colicky abdominal pain after laparoscopic Roux-en Y gastric bypass (LRYGB), even in the absence of other symptoms, laboratory and/or radiologic abnormalities. Parakh et al. [30] suggested observation when CT scan was non-diagnostic and abdominal pain improved. Champion et al. [28] recommended surgical exploration when colicky pain persisted or recurred after LRYGB, even if CT finding was non-diagnostic. In our center, the patients without symptoms and bowel ischemia on CT underwent non-surgical management.
There were several limitations to this study. First, it was retrospective; thus, the distribution of background characteristics of the study population was not uniform. Second, since work-up for internal hernia was performed mainly with CT, there are possibilities that the patients, who were asymptomatic without follow-up CT or had false-negative CT findings, were underdiagnosed, leading to an inaccurate estimation of the incidence of internal hernia. Third, this study was conducted at a single center and was limited to the Korean population; thus, our results might not be generalizable to other ethnicities.
Conclusions
Internal hernia after gastrectomy for gastric cancer is a significant complication because of the possibility of severe morbidities and mortality. The closure of the jejunojejunostomy mesenteric, Petersen’s, and transverse colon mesenteric defects using a nonabsorbable continuous suture is recommended for patients undergoing gastrectomy for gastric cancer to reduce the incidence of internal hernia.
References
Rosin D, Zmora O, Hoffman A, Khaikin M, Bar Zakai B, Munz Y, et al. Low incidence of adhesion-related bowel obstruction after laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech A. 2007;17:604–7.
Dowson HM, Bong JJ, Lovell DP, Worthington TR, Karanjia ND, Rockall TA. Reduced adhesion formation following laparoscopic versus open colorectal surgery. Br J Surg. 2008;95:909–14.
Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. 2014;259:109–16.
Miyagaki H, Takiguchi S, Kurokawa Y, Hirao M, Tamura S, Nishida T, et al. Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J Surg. 2012;36:851–7.
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N, Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72.
Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case–control study. Ann Surg Oncol. 2009;16:1507–13.
Paroz A, Calmes JM, Giusti V, Suter M. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a continuous challenge in bariatric surgery. Obes Surg. 2006;16:1482–7.
Renvall S, Niinikoski J. Internal hernias after gastric operations. Eur J Surg. 1991;157:575–7.
Hardy JD. Jackson, Mississippi. Problems associated with gastric surgery: a review of 604 consecutive patients with annotation. Am J Surg. 1964;108:699–716.
Himpens J, Verbrugghe A, Cadiere GB, Everaerts W, Greve JW. Long-term results of laparoscopic Roux-en-Y gastric bypass: evaluation after 9 years. Obes Surg. 2012;22:1586–93.
Aghajani E, Jacobsen HJ, Nergaard BJ, Hedenbro JL, Leifson BG, Gislason H. Internal hernia after gastric bypass: a new and simplified technique for laparoscopic primary closure of the mesenteric defects. J Gastrointest Surg. 2012;16:641–5.
Bauman RW, Pirrello JR. Internal hernia at Petersen’s space after laparoscopic Roux-en-Y gastric bypass: 6.2% incidence without closure—a single surgeon series of 1047 cases. Surg Obes Relat Dis. 2009;5:565–70.
Hosoya Y, Lefor A, Ui T, Haruta H, Kurashina K, Saito S, et al. Internal hernia after laparoscopic gastric resection with antecolic Roux-en-Y reconstruction for gastric cancer. Surg Endosc. 2011;25:3400–4.
Kelly KJ, Allen PJ, Brennan MF, Gollub MJ, Coit DG, Strong VE. Internal hernia after gastrectomy for cancer with Roux-Y reconstruction. Surgery. 2013;154:305–11.
Yoshikawa K, Shimada M, Kurita N, Sato H, Iwata T, Higashijima J, et al. Characteristics of internal hernia after gastrectomy with Roux-en-Y reconstruction for gastric cancer. Surg Endosc. 2014;28:1774–8.
Kim YW, Baik YH, Yun YH, Ho NB, Hyun KD, Ju CI, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7.
Gunabushanam G, Shankar S, Czerniach D, Kelly JJ, Perugini RA. Small-bowel obstruction after laparoscopic Roux-en-Y gastric bypass surgery. J Comput Assist Tomogr. 2009;33:369–75.
Hwang RF, Swartz DE, Felix EL. Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc. 2004;18:1631–5.
Kojima K, Inokuchi M, Kato K, Motoyama K, Sugihara K. Petersen’s hernia after laparoscopic distal gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer. 2014;17:146–51.
Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, et al. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–55.
Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, et al. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer. 2011;15:198–205.
Comeau E, Gagner M, Inabnet WB, Herron DM, Quinn TM, Pomp A. Symptomatic internal hernias after laparoscopic bariatric surgery. Surg Endosc. 2005;19:34–9.
Muller MK, Rader S, Wildi S, Hauser R, Clavien PA, Weber M. Long-term follow-up of proximal versus distal laparoscopic gastric bypass for morbid obesity. Br J Surg. 2008;95:1375–9.
Hope WW, Sing RF, Chen AY, Lincourt AE, Gersin KS, Kuwada TS, et al. Failure of mesenteric defect closure after Roux-en-Y gastric bypass. JSLS. 2010;14:213–6.
Papasavas PK, Caushaj PF, McCormick JT, Quinlin RF, Hayetian FD, Maurer J, et al. Laparoscopic management of complications following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:610–4.
Kaouk JH, Haber GP, Goel RK, Desal MM, Aron M, Rackley RR, et al. Single-port laparoscopic surgery in urology: initial experience. J Urol. 2008;71:3–6.
Steele KE, Prokopwicz GP, Magnuson T, Lidor A, Schweitzer M. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc. 2008;22:2056–61.
Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:596–600.
Lannelli A, Facchiano E, Gugenheim J. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265–71.
Parakh S, Soto E, Merola S. Diagnosis and management of internal hernias after laparoscopic gastric bypass. Obes Surg. 2007;17:1498–502.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The need for informed consent was waived because of the retrospective nature of the study design.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kang, K.M., Cho, Y.S., Min, SH. et al. Internal hernia after gastrectomy for gastric cancer in minimally invasive surgery era. Gastric Cancer 22, 1009–1015 (2019). https://doi.org/10.1007/s10120-019-00931-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-019-00931-1