Abstract
Background
To decrease the incidence of internal hernia after laparoscopic Roux-en-Y gastric bypass, recent recommendations indicated closure of mesenteric defects and Petersen’s defect. Laparoscopic distal gastrectomy for gastric cancer is used increasingly, so the incidence of Petersen’s hernia can also increase, but the trend has not been studied.
Methods
This study retrospectively reviewed 358 consecutive patients who underwent laparoscopic distal gastrectomy for gastric cancer at one institution, with antecolic Roux-en-Y (RY) reconstruction.
Results
Petersen’s hernia occurred in 6 (2.2 %) of 268 patients whose Petersen’s defect was not closed by a mean of 351 days after surgery. All the patients underwent reoperation with reduction and repair of the hernia except the first case. In 90 subsequent cases, with closure of the Petersen’s defect, internal hernias did not occur (0/90 cases; p = 0.06). Focusing on the totally laparoscopic procedure, Petersen’s hernia occurred in 2 (5.1 %) of 39 patients, whereas in 81 subsequent cases, with closure of Petersen’s defect, internal hernias did not occur (0/81 cases; p = 0.03).
Conclusions
Based on the recent recommendations for bariatric surgery, closure of this potential hernia defect is necessary after laparoscopic distal gastrectomy with R-Y reconstruction for gastric cancer.
Similar content being viewed by others
Introduction
Laparoscopic distal gastrectomy has been one of the standard treatments of choice for patients with early gastric cancer, not only in Asia but also in Western countries, with numerous clinical series being reported [1–3]. In Japan, Billroth-I (B-I) reconstruction has typically been performed after laparoscopic distal gastrectomy, but because of high rates of remnant gastritis after B-I reconstruction, Roux-en-Y (R-Y) reconstruction has gradually gained favor for use and has been one of the standard methods of reconstruction after laparoscopic distal gastrectomy [4–6].
Internal hernia has been recognized as a potential complication of R-Y gastric bypass (RYGBP), with an incidence of 1–5 % in open surgery during the past decade [7]. Studies for complications after RYGBP have reported a significantly higher incidence of long-term internal hernia after laparoscopic surgery than open surgery [7–10].
In gastric cancer patients who undergo laparoscopic distal gastrectomy with R-Y reconstruction, which includes a reconstruction procedure similar to RYGBP, the incidence of internal hernia might be higher after laparoscopic surgery than after open surgery as if reported after bariatric surgery. In the recent widespread use of laparoscopic surgery for gastric cancer, we should be aware of internal hernia after distal gastrectomy. However, there are only a few reports on the incidence of internal hernia, especially Petersen’s hernia, with R-Y reconstruction after laparoscopic distal gastrectomy for cancer [11, 12]. This is a matter of concern because these hernias can present dramatically as an acute bowel obstruction with necrosis of the bowel, especially when diagnosis with prompt surgical intervention is delayed [9].
We report our experience with Petersen’s hernia since the introduction of laparoscopic distal gastrectomy with antecolic R-Y reconstruction, placing our focus on approaches to reduce internal hernia after laparoscopic gastric cancer surgery.
Patients and methods
Patients
Patients with gastric cancer who underwent laparoscopy-assisted distal gastrectomy (LADG) or totally laparoscopic distal gastrectomy (TLDG) at Tokyo Medical and Dental University Hospital were retrospectively reviewed from the medical records. Indication for the LADG was based on the preoperatively determined depth of tumor invasion (T2 and T3, which are according to the TNM classification, 7th edition, as classified by the American Joint Committee on Cancer and the Union for International Cancer Control), absence of metastatic lymph nodes, and the clear presence of an adenocarcinoma in the lower two thirds of the stomach, regarded as an indication for distal gastrectomy.
Laparoscopic distal gastrectomy
Five ports (three 12-mm and two 5-mm ports) were placed, a flexible laparoscope was introduced through the umbilical port with the patient under general anesthesia, and carbon dioxide (CO2) pneumoperitoneum established. Laparoscopic mobilization of the stomach and lymph node dissection were performed. The duodenum was divided distal to the pylorus with an endoscopic linear stapler (Echelon60; Ethicon, OH, USA).
Next, the stomach was divided with endoscopic linear staplers followed by a routine regional lymph node dissection.
LADG
The specimen was retrieved through a 4- to 6-cm incision in the epigastria region. On the distal side 20 cm distal to the ligament of Treitz, the mesentery of the jejunum was divided for a distance of 10 cm. The jejunum then was divided with an endoscopic linear stapler. The distal jejunum was brought up to the remnant stomach in an antecolic fashion. The greater curvature of the stomach was anastomosed to the jejunum side-to-side with a stapler. The site of entry for the linear stapler then was closed using a hand-sewn suture. The jejuno-jejunostomy was fashioned using an endoscopic linear stapler through the mini-laparotomy in a conventional fashion under direct vision.
Totally laparoscopic distal gastrectomy (TLDG)
R-Y reconstruction involves dividing the jejunum 25–30 cm distal to the ligament of Treitz using the 60-mm endoscopic linear stapler. A small incision is made in the anti-mesenteric site of jejunum before it is brought up the antecolic route, and a side-to-side anastomosis is constructed with the greater curvature of the stomach. The “common entry hole’’ of the gastrojejunostomy is closed by applying the same endoscopic linear stapler in a different plane. Finally, the proximal end of the jejunum is reattached to the remainder of the intestine 30 cm distal to the initial division using a similar technique (Fig. 1).
In all the patients we report, the jejunojejunal mesenteric defect was closed during the procedure under direct vision using a 2-0 nonabsorbable running suture (Ethibond; Ethicon) or 4-0 absorbable intermittent sutures (Vicryl; Ethicon), and sometimes under laparoscopic vision in the case of obese patients, at the discretion of the operating surgeon.
All the patients underwent routine computed tomography (CT) scan or abdominal ultrasonography (US) 6 months postoperatively as part of the routine evaluation. Patients who underwent reoperation because of symptoms and CT findings consistent with internal hernia were reviewed. There were no internal hernia patients who were diagnosed by CT scan without symptoms. The patients who experienced internal hernia were reviewed in two groups. The no-closure group (initial 268 patients) was compared with the group that underwent routine closure of Petersen’s defect (subsequent 90 patients).
Results
In this study, 358 patients were reviewed from January 2004 to June 2012. The no-closure group included 268 patients (229 LADG and 39 TLDG), and the closure group included 90 patients (81 TLDG and 9 LADG). Six patients underwent surgical treatment of internal hernia after gastrectomy for gastric cancer, including 2 women and 4 men, aged 47–73 years (median, 59.5 years). Internal hernia occurred for 6 (2.2 %) of the 268 patients in the no-closure group. The mean observation period was 1,545 days (range, 891–3,111) in the no-closure group. The mean time to diagnosis of internal hernia was 351 days (range, 2–678) (Table 1). We analyzed the clinical data between the patients with and without Petersen’s hernia regarding sex, age, body mass index (BMI), type of procedure, operating time, blood loss, and stage. Univariate analysis revealed that there is no risk factor associated with Petersen’s hernia.
Except the first case, all the other internal hernia patients presented with colicky pain. The radiologic findings were abnormal for the patients, with dilation of the small bowel, remnant stomach, or both, and twisted appearance of the mesentery and its vessels, suggesting a volvulus (Fig. 2). Emergency surgical treatment comprising reduction of the hernia and closure of Petersen’s defect was performed in an open fashion because of the dilated intestine, except in the first case. No adhesions were noted at reoperation for any of the patients. The first case was treated under laparoscopic procedure comprising reduction of the hernia without closure of the Petersen’s hernia (Fig. 3a, b).
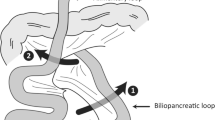
All hernias were located at Petersen’s defect, which is the space between the Roux limb and the transverse mesocolon (Fig. 4). No further cases of internal hernia were recorded after the surgical practice was changed to include routine closure of Petersen’s defect using a 2-0 nonabsorbable running suture.
No severe complications occurred after LADG. One patient in the non-closure group required reoperation because of leakage at gastrojejunostomy after TLDG. This series had no mortality.
Discussion
During the past decade, laparoscopic distal gastrectomy has gained acceptance and many studies have shown several advantages over the open approach to the same surgery, such as reduction in postoperative pain and earlier resumption of normal activities. Several randomized controlled studies of LADG showed further benefits including less bleeding and improved quality of life compared with a traditional open distal gastrectomy [1–3].
In Japan, B-I reconstruction has typically been performed after laparoscopic distal gastrectomy. Therefore, the incidence of internal hernia after laparoscopic distal gastrectomy was quiet low. Because of high rates of remnant gastritis after B-I reconstruction, R-Y reconstruction has gradually gained favor for use and has been one of the standard methods of reconstruction after laparoscopic distal gastrectomy [4–6].
In gastric cancer patients who undergo laparoscopic distal gastrectomy with R-Y reconstruction, there exist Petersen’s defect, which is the space between the Roux limb and the transverse mesocolon. Thus, there is the possibility of internal hernia after laparoscopic distal gastrectomy with R-Y reconstruction. Laparoscopic RYGBP reportedly has a high rate of postoperative internal hernia with an incidence of 3.1 to 9.7 % in laparoscopic surgery [7–9]. These authors reported 7.9 to 27 % of internal hernias occurred at Petersen’s defect after laparoscopic RYGBP, with a high bowel resection rate and a mortality rate [7–9]. The reason why the incidence of internal hernia is higher in laparoscopic RYGBP may be that laparoscopy decreases tissue trauma and therefore causes fewer adhesions than open surgery [9].
Body weight loss may be another risk factor for patients with a postgastrectomy internal hernia. Miyagaki et al. [12] reported the reduction (13.5 %) after distal gastrectomy in their study was larger than in other studies (range, 9.1–10.5 %). In our study, the reduction after distal gastrectomy was 11.4 % on average (data not shown), which was also slightly larger than in other studies [13, 14]. Weight loss is considered a risk factor for internal hernia in RYGBP [15, 16]. The large decrease in mesenteric fat after distal gastrectomy is a major cause of weight loss after surgery [17, 18]. As a result, a large weight loss may increase the size of the mesenteric defect, thus accelerating the internal hernia [19].
As for the position of the Roux limb, the retrocolic route in laparoscopic RYGBP has three potential sites for internal hernia, namely, the transverse colon mesentery, Petersen’s defect, and the enteroenterostomy site [8–10]. Recently, many authors have preferred the antecolic route for the Roux limb, which reduces the potential sites for hernia from three to two, including Petersen’s defect after a laparoscopic RYGBP [10, 20, 21]. As for the route, the antecolic route may be better because it eliminated one of the most common sites for herniation, the mesocolon, as for laparoscopic distal gastrectomy with R-Y reconstruction.
Regarding prevention of internal hernia after laparoscopic distal gastrectomy, closure of the mesenteric defect and Petersen’s space has reported a declined incidence of internal hernias from the studies of bariatric surgery [8, 15]. Moreover, Paroz et al. [7] recommended routine closure of all mesenteric defects using a running nonabsorbable suture to prevent internal hernia after laparoscopic RYGBP (internal hernia ratio: 5.6 % absorbable to 1.3 % nonabsorbable; p = 0.03). We have performed a 2-0 nonabsorbable running suture to close Petersen’s defect after experiencing several cases of Petersen’s herniation, and no more such events have happened.
Hosoya et al. [11] first reported internal herniation after laparoscopy-assisted gastrectomy with gastric cancer patients. In their report, they experienced 4 (7 %) jejunojejunal hernias among 58 patients whose jejunojejunal mesenteric defect was not closed. They did not close Petersen’s defect, and they never experienced an internal hernia of Petersen’s defect, perhaps because they performed reconstruction under direct vision through mini-laparotomy. Actually, we performed reconstruction through mini-laparotomy until December 2008. We experienced 4 (1.7 %) Petersen’s hernia cases of 229 LADG patients whose jejunojejunal mesenteric defect was closed with interrupted absorbable sutures, which is significantly lower than TLDG patients (5.1 %). Manipulation of the intestines under direct vision increases adhesion, which may decrease the internal herniation after LADG. Therefore, we must take care to prevent internal hernia, especially after TLDG. It appears from the current series that closure of Petersen’s defect may be very important in reducing the incidence of internal hernia.
The use of laparoscopic distal gastrectomy followed by R-Y reconstruction for the treatment of gastric cancer is increasing rapidly and probably will continue to increase in the future. Also, TLDG after laparoscopic distal gastrectomy is the trend for gastric cancer patients. Closure of the jejunojejunal defect and Petersen’s defect with continuous nonabsorbable running suture is recommended for these patients, consistent with the recommendations for laparoscopic RYGBP with an antecolic R-Y reconstruction, to minimize the incidence of internal hernia.
References
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N, Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72.
Kim YW, Baik YH, Yun YH, Ho NB, Hyun KD, Ju CI, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7.
Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case–control study. Ann Surg Oncol. 2009;16:1507–13.
Takaori K, Nomura E, Mabuchi H, Lee SW, Agui T, Miyamoto Y, et al. A secure technique of intracorporeal Roux-Y reconstruction after laparoscopic distal gastrectomy. Am J Surg. 2005;189:178–83.
Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg. 2008;247:962–7.
Fukuhara K, Osugi H, Takada N, Takemura M, Higashino M, Kinoshita H. Reconstructive procedure after distal gastrectomy for gastric cancer that best prevents duodenogastroesophageal reflux. World J Surg. 2002;26:1452–7.
Paroz A, Calmes JM, Giusti V, Suter M. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a continuous challenge in bariatric surgery. Obes Surg. 2006;16:1482–7.
Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13:350–4.
Capella RF, Iannace VA, Capella JF. Bowel obstruction after open and laparoscopic gastric bypass surgery for morbid obesity. J Am Coll Surg. 2006;203:328–35.
Steele KE, Prokopowicz GP, Magnuson T, Lidor M, Schweitzer M. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc. 2008;22:2056–61.
Hosoya Y, Lefor A, Ui T, Haruta H, Kutrashina K, Saito S, et al. Internal hernia after laparoscopic gastric resection with antecolic Roux-en-Y reconstruction for gastric cancer. Surg Endosc. 2001;25:3400–4.
Miyagaki H, Takiguchi S, Kurokawa Y, Hirao M, Tamura S, Nishida T, et al. Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J Surg. 2012;36:851–7.
Nunobe S, Okaro A, Sasako M, Saka M, Fukagawa T, Katai H, et al. Billroth I versus Roux-en-Y reconstructions: a quality-of-life survey at 5 years. Int J Clin Oncol. 2007;12:433–9.
Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, et al. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer. 2011;15:198–205.
Comeau E, Gagner M, Inabnet WB, Herron DM, Quinn TM, Pomp A. Symptomatic internal hernias after laparoscopic bariatric surgery. Surg Endosc. 2005;19:34–9.
Muller MK, Rader S, Wildi S, Hauser R, Clavien PA, Weber M. Long-term follow-up of proximal versus distal laparoscopic gastric bypass for morbid obesity. Br J Surg. 2008;95:1375–9.
Hope WW, Sing RF, Chen AY, Lincourt AE, Gersin KS, Kuwada TS, et al. Failure of mesenteric defect closure after Roux-en-Y gastric bypass. JSLS. 2010;14:213–6.
Miyato H, Kitayama J, Hidemura A, Ishigami H, Kaisaki S, Nagawa H. Vagus nerve preservation selectively restores visceral fat volume in patients with early gastric cancer who underwent gastrectomy. J Surg Res. 2012;173:60–7.
Papasavas PK, Caushaj PF, McCormick JT, Quinlin RF, Hayetian FD, Maurer J, et al. Laparoscopic management of complications following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2002;12:559–63.
Hwang RF, Swartz DE, Felix EL. Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc. 2004;18:1631–5.
Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:596–600.
Acknowledgments
We have received no grants for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kojima, K., Inokuchi, M., Kato, K. et al. Petersen’s hernia after laparoscopic distal gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer 17, 146–151 (2014). https://doi.org/10.1007/s10120-013-0256-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-013-0256-8