Abstract
Background
We compared efficacy and safety of paclitaxel/capecitabine therapy followed by capecitabine for maintenance (PACX) versus cisplatin/capecitabine therapy (XP) in advanced gastric cancer.
Methods
Multicenter, randomized, phase III trial was conducted in China (December 2009–February 2014). Adults (n = 320) with histologically confirmed, untreated metastatic/unresectable gastric or gastroesophageal junction adenocarcinoma; with ≥ 1 measureable lesions according to Response Evaluation Criteria in Solid Tumors 1.0 criteria; Karnofsky performance score ≥ 70 and life expectancy ≥ 3 months were randomized (1:1) to PACX or XP. PACX group received paclitaxel 80 mg/m2 intravenous on days 1 and 8; capecitabine 1000 mg/m2 orally BD on days 1–14, followed by a 7-day rest interval for 4 cycles, followed by maintenance capecitabine at same dosage/schedule until disease progression, unendurable adverse events or death. XP group received cisplatin intravenous 80 mg/m2 on day 1 and capecitabine at same dosage/schedule as PACX group per cycle for 6 cycles.
Results
Median progression-free survival (5.0 versus 5.3 months; hazard ratio [95% CI]: 0.906; 0.706–1.164; p = 0.44) and overall survival (12.5 versus 11.8 months; hazard ratio: 0.878 [0.685–1.125]; p = 0.30) were not significantly different between PACX and XP groups. Objective response rate was significantly higher (43.1 versus 28.8%; p = 0.012) and disease control rate was similar (77.5 versus 72.5%; p = 0.75) in PACX versus XP, respectively. Quality of life was significantly improved in PACX versus XP after three treatment cycles. Many treatment-related adverse events were significantly lesser in PACX than XP.
Conclusions
First-line chemotherapy with PACX is effective with milder toxicities in advanced gastric cancer, but could not replace XP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the third most common cancer and the second leading cause of cancer deaths globally. Approximately 50% of the incident cases occur in Eastern Asia (mainly in China) [1]. The median survival of patients with advanced gastric cancer (AGC) is 7–10 months in a majority of large clinical studies [2].
Compared with best supportive care alone, palliative chemotherapy (CT) improves survival and quality of life (QoL) for patients with AGC [2, 3]. In different countries, fluorouracil and cisplatin combination with or without a third drug are most widely used [4]. In the ToGA study, combination therapy of the anti-human epidermal growth factor receptor 2 (HER2) antibody, trastuzumab, with 5-fluorouracil or capecitabine plus cisplatin showed significantly improved survival compared with CT alone in patients with HER2-positive late-stage gastric cancer [5]. However, the toxicities and disadvantages related with use of cisplatin highlight the need for alternative therapeutic options that would maintain good QoL during CT.
Taxanes are considered as alternative first-line chemotherapy options for AGC [4], since they improve survival without compromising QoL. Paclitaxel shows good efficacy and tolerance in AGC either as monotherapy or in combination with other CT drugs [6]. Capecitabine, an oral fluoropyrimidine is rapidly converted to 5-fluorouracil (5-FU) in tumor tissue, and is becoming popular and replacing fluorouracil in AGC for convenience and satisfactory efficacy [7,8,9]. The randomized phase III non-inferiority trial ML17032 demonstrated that capecitabine can replace 5-FU in AGC combination therapy [10]. Synergistic efficacy mediated by taxane-induced upregulation of thymidine phosphorylase and minimal overlap of major toxicities are advantages of capecitabine and taxane combination [11].
Maintenance therapy with a simplified drug regimen following an intensive CT reduced adverse effects without compromising survival benefit in advanced colorectal cancer [12].
In our previous phase II study, paclitaxel and capecitabine combination as first-line CT with capecitabine maintenance after disease control showed promising efficacy and good tolerance, with median PFS of 188 days and median OS of 354 days, and the median OS in the patient subgroup maintained with capecitabine monotherapy was 531 days [13]. Further randomized trials are required to accurately evaluate the efficacy and safety of this promising regimen for the AGC treatment.
The purpose of this prospective, randomized, controlled phase III study was to compare the efficacy and safety of paclitaxel plus capecitabine regimen as a first-line CT followed by capecitabine monotherapy (PACX) as a maintenance therapy versus cisplatin plus capecitabine (XP) regimen for AGC.
Methods
Study design
This was a multicenter, open-label, active-controlled phase III trial conducted in 22 national hospitals with specialized cancer centers across different regions of China from December 2009 to February 2014 (Clinicaltrials.gov NCT01015339: https://clinicaltrials.gov/ct2/show/NCT01015339).
Patients
Eligible participants were aged ≥ 18 years with histologically confirmed gastric or gastroesophageal junction (GEJ) adenocarcinoma; previously untreated metastatic or unresectable disease; one or more measureable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 criteria; Karnofsky performance score (KPS) ≥ 70; life expectancy of ≥ 3 months; and acceptable results of the pre-specified hematological and biochemical tests. Previous neo-adjuvant or adjuvant treatments for gastric cancer (except taxanes) were permissible if completed > 6 months, or > 1 year if they comprised capecitabine (not more than 2 cycles) and/or cisplatin (total dose not more than 300 mg/m2) before study initiation. No prior radiotherapy was permitted, except for non-target lesions if completed > 4 weeks before study initiation. Key exclusion criteria included brain metastasis, long-term systemic steroid treatment, significant clinical symptoms of cardiac diseases within the last 6 months, known allergy to any study drugs, inability to receive oral medication, pregnancy or lactation period, use of any investigational agent within the past 28 days, any other previous malignancy within 5 years except non-melanoma skin cancer or in situ cervix carcinoma, and issues due to legal incapacity.
Procedures
Eligible patients were randomly assigned in a 1:1 ratio using a SAS 9.1.3 generated sequence. Stratified randomization by minimization was performed based on KPS (≥ 80/< 80), resection of primary tumor (performed/not performed), weight loss within last 3 months (≥ 5%/< 5%), primary tumor site at the GEJ (yes/no). Nurses dispersed the drugs according to the randomization list.
In PACX group, a total of 4 cycles PACX therapy followed by capecitabine monotherapy for maintenance was administered. For each cycle, paclitaxel was administered intravenously at 80 mg/m2 over 3 h on days 1 and 8; capecitabine (Xeloda; F. Hoffmann-La Roche Ltd., Basel, Switzerland) was administered orally at 1000 mg/m2 twice daily on days 1–14, followed by a 7-day rest interval (Online Resource 1). Patients received standard anti-hypersensitivity prophylaxis treatment including 10 mg of intravenous dexamethasone, 40 mg of intramuscular diphenhydramine, and 400 mg of intravenous cimetidine 30 min before each paclitaxel administration. After this double-drug regimen, patients received capecitabine monotherapy at the same dosage and schedule as the maintenance therapy until disease progression or development of unendurable adverse events (AEs) or death.
In XP group, a total of 6 cycles XP therapy were planned. Cisplatin was administered intravenously at 80 mg/m2 for 2 h on day 1 with hydration and standard delayed emesis prophylaxis treatment per cycle. Capecitabine was administered at the same dosage and schedule as in PACX group (Online Resource 1). In both groups, cycles were repeated every 3 weeks until progression, occurrence of unendurable AEs, consent withdrawal, or a total of 4 or 6 cycles of therapy for PACX and XP, respectively, whichever was earlier.
Dose adjustment was based on the severity of hematologic and non-hematologic toxicity, graded according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) and the pre-decided dose modification scheme as specified in the study protocol. Simultaneous dose reduction of capecitabine and paclitaxel was avoided unless patients developed severe AEs.
Routine evaluation (physical examination, vital signs, KPS, laboratory hematological and/or serum chemistry, recording and grading of AEs) of patients was conducted on a weekly basis during therapy. Tumor response was evaluated by computerized tomography or magnetic resonance imaging every 6 and 9 weeks during treatment and follow-up, respectively, according to the RECIST 1.0 criteria. QoL was evaluated using the European Organization for Research and Treatment of Cancer QoL Questionnaire (EORTC QLQ)-C30 and EORTC QLQ-STO22 questionnaires at the beginning of each CT cycle until progression, occurrence of unendurable AEs, consent withdrawal, or completion of a total 4 or 6 cycles of therapy for PACX and XP, respectively, whichever was earlier. After disease progression, patients were followed-up every 12 weeks to determine survival until death or the completion of the study.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the duration from the date of signing informed consent forms to the first observed disease progression (per imaging examination) or any-cause death, whichever was earlier. The secondary endpoints included disease control rate (DCR) representing the summation of complete response (CR), partial response (PR), and stable disease (SD) rates; objective response rate (ORR), the proportion of total tumor responses (CR and PR) relative to treated patients; overall survival (OS), the duration from the date of signing informed consent forms to death; AEs; serious AEs (SAEs) and QoL.
Statistical analysis
An overall sample size of 320 subjects (160 in each group) will achieve 80% power at a 0.05 significance level to detect a hazard ratio of 0.69 when the control group median PFS is 4.5 months.
Safety analysis was performed on the safety set (SS) including all patients who had received treatment at least once, and efficacy analysis was performed on intention-to-treat (ITT) set including all patients who intended to receive treatment. An interim safety analysis was scheduled after enrolment of 160 cases. The final analysis of all the endpoints was performed 12 months after the last treatment of the last patient or after approximately 75% of patients had died, whichever occurred first.
PFS and OS were assessed using the Kaplan–Meier method and compared by the log-rank test. ORR and DCR were presented with the corresponding two-sided 95% confidence interval (CI) and analyzed by logistic regression. AEs, SAEs, and drug dose exposure were analyzed using descriptive methods. The p values were two-sided and p < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS (version 9.1.3).
Results
Patient characteristics
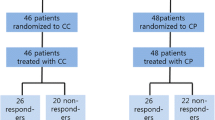
A total of 320 patients were enrolled and randomized in 1:1 ratio to PACX or XP groups (Fig. 1). A total of 252 (78.8%) deaths occurred and 8 patients were lost during follow-up in each arm. The ITT set included 160 patients from each treatment group. The SS set comprised 157 and 148 patients from PACX group and XP group, respectively. The baseline characteristics were well-balanced in both groups (Table 1).
The median treatment cycle for the double-drug regimen was 4 and 5 cycles in PACX and XP groups, respectively. The median percentage of actual dose administered relative to the planned dose was > 80% for capecitabine in both groups, 96.8% for paclitaxel in PACX group, and 98% for cisplatin in XP group. In total, 26 (16.6%) and 40 (27.0%) patients had pac2litaxel and cisplatin dose adjustment (p = 0.02), 31 (19.7%) and 40 (27.0%) patients had capecitabine dose adjustment (p = 0.12), and drug administration was postponed in 101 (64.3%) and 108 (73.0%) patients (p = 0.07), in PACX and XP group, respectively. In PACX group, 61 (38.9%) patients did not receive capecitabine monotherapy: n = 37, disease progression; n = 10, refusal to maintain; n = 8, unknown reasons; n = 5, poor tolerance; n = 1, surgery. The remaining 96 patients in PACX group received capecitabine monotherapy, and the median number of maintenance cycles was 4. The proportion of patients receiving second-line therapy was similar in both groups (PACX group: n = 56, 35.7%; XP group: n = 47, 31.8%; p = 0.465). The second-line treatment regimens in both groups were similar (p = 0.423). 42.3% (24/56) patients in PACX group received oxaliplatin-based chemotherapy, while 30.0% (14/47) patients in XP group received docetaxel-based chemotherapy.
Efficacy
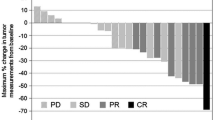
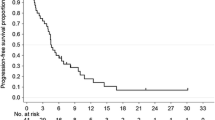
The median follow-up time was 31.4 (95% CI 27.7–35.8) months. Median PFS, the primary endpoint of this study, was 5.0 (95% CI 4.3–6.3) months in PACX group and 5.3 (95% CI 4.7–5.8) months in XP group (hazard ratio 0.906; 95% CI 0.706–1.164; p = 0.44, Fig. 2a). Median OS was 12.5 (95% CI 11.5–14.5) months in PACX group and 11.8 (95% CI 10.0–13.7) months in XP group (hazard ratio 0.878; 95% CI 0.685–1.125; p = 0.30, Fig. 2b). ORR was significantly higher in PACX group than XP group (43.1% versus 28.8%, p = 0.012), whereas DCR (77.5 versus 72.5%, p = 0.75) was not significantly different (Table 2). Median PFS, median OS, ORR, and DCR were not significantly different between both groups for subgroup analysis of patients with different Lauren classification and different types of metastases.
Progression-free survival and overall survival. a Progression-free survival was not significantly different; b overall survival was not significantly different. CI confidence interval, PACX combination therapy of paclitaxel and capecitabine followed by capecitabine monotherapy as maintenance therapy, XP cisplatin and capecitabine combination therapy
Safety and quality of life
Forest plot of time to first deterioration assessed by EORTC QLQ-C30 for PACX group was significantly shorter than that of XP group, suggesting significantly improved QoL in PACX group than in XP group (Fig. 3a). The EORTC QlQ-STO22 was also significantly different as to some symptoms between both groups (Fig. 3b).
Quality of life: time to first deterioration (ITT population). a Forest plot of EORTC QLQ-C30 using COX analysis (ITT population); b forest plot of EORTC QLQ-ST022 using COX analysis (ITT population). CI confidence interval, EORTC QLQ European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, HR hazard ratio, ITT intention-to-treat, PACX combination therapy of paclitaxel and capecitabine followed by capecitabine monotherapy as maintenance therapy, XP cisplatin and capecitabine combination therapy
During the study, 1 patient in XP group erroneously received the treatment regimen of PACX group. Thus, safety was evaluated in 158 patients of PACX group and 147 patients of XP group. Blood and lymphatic system disorders and gastrointestinal system disorders were the most common treatment-related AEs in both groups. The incidences of treatment-related leukopenia, thrombocytopenia, nausea, vomiting, lack of appetite, and vascular disorders were significantly higher in XP group than PACX group (all p < 0.05, Table 3). Alopecia and musculoskeletal and connective tissue disorders were more frequent in PACX group than in XP group (all p < 0.05, Table 3). Analysis of treatment-related AEs at level III or IV revealed that the incidences of anemia, thrombocytopenia, vomiting and nausea were significantly higher in XP group than PACX group (all p < 0.05, Table 3). 6 (3.8%) patients in PACX group and 4 (2.7%) patients in XP group developed treatment-related SAEs (Table 3).
Discussion
In this randomized phase III study, PACX regimen did not show longer PFS, OS, and DCR, but significantly higher ORR and QoL compared with XP regimen. In spite of the negative result of efficacy, the PACX regimen was related with significantly lower incidences of hematologic toxicity such as leukopenia and thrombocytopenia and gastrointestinal AEs such as nausea, vomiting and lack of appetite, than the XP regimen.
Although PFS is not always strictly related with OS in first-line therapy of AGC, it is often used as an alternative, especially when progression is likely to be related to symptomatology. Also, considering convenience and economical factors, we selected PFS as the primary endpoint. Capecitabine monotherapy was introduced in PACX regimen after disease control; we thus supposed PFS might be prolonged by continual exposing of chemotherapeutical agent compared with fixed cycles of XP. This study was planned as a superior design, but not as a non-inferior design.
The efficacy and safety of combination of paclitaxel and capecitabine as a first-line treatment for AGC have been investigated in previous phase II trials. The results of the current study, which showed a median PFS of 5.0 months and a median OS of 12.5 months in PACX group, were similar to previous phase II clinical trial findings with capecitabine maintenance as well [13, 14], but longer than that of Yuan’s retrospective data [15]. Since the number of patients having second-line and follow-up treatments are not statistically different between two groups, the longer median PFS and OS in this study compared with Yuan’s data might be related with the addition of capecitabine monotherapy following the double-drug combination therapy, indicating that single-drug monotherapy following a first-line CT for AGC might improve patient survival.
Preclinical studies have demonstrated that combination of capecitabine and paclitaxel had synergistic antitumor activity [16, 17]. Capecitabine is finally converted to fluorouracil by thymidine phosphorylase, which is expressed at higher levels in tumor tissue, leading to the accumulation of fluorouracil in tumor tissue [18, 19]. Sequential exposure to paclitaxel following 5-FU exerted additive cytotoxic effects in human carcinoma cell lines by up-regulating thymidine phosphorylase activity [16, 17]. For the first time, in this current randomized control trial, we found that combination therapy of paclitaxel and capecitabine followed by capecitabine monotherapy resulted in significantly higher ORR than combination therapy of cisplatin and capecitabine for AGC. Despite higher ORR, PACX regimen showed similar PFS, OS, and DCR with XP regimen in advanced gastric cancer. This might primarily be due to its genetic complexity and heterogeneity. Therefore, the identification of novel and specific markers to predict PACX treatment in gastric cancer is important.
The safety profile evaluated in previous studies consistently shows that combination of paclitaxel and capecitabine as a first-line therapy for AGC was related with mild AEs, and the most common grade 3–4 AEs included neutropenia, leukopenia, and vomiting [13,14,15]. In this study, comparison of the safety profile revealed that the PACX regimen was associated with significantly reduced incidences of blood and lymphatic system disorders and gastrointestinal system disorders compared with the XP regimen. Consistently, QoL was also significantly improved in patients receiving PACX regimen than those receiving XP regimen.
It can be argued that 4 cycles of combination therapy in PACX are not enough, thus limiting the efficacy and explaining the better tolerance. It is not known whether more cycles of paclitaxel combined with capecitabine before monotherapy maintenance could further improve survival. In future clinical practice and clinical study, it is at least reasonable to continue combination therapy until accumulated peripheral neuropathy.
To our knowledge, this study is the first multicenter phase III randomized control trial to compare the efficacy and safety of PACX regimen. To maximally balance patient clinical characteristics between both groups at randomization, we used four stratification factors (KPS, previous resection of primary tumor, weight loss within 3 months of enrolment, and primary tumor site). The well-balanced baseline data of this study suggested that our randomization strategy was effective. However, there are limitations in this study. The number of patients (96/160, 60%) in PACX group who received capecitabine maintenance therapy was relatively small. Moreover, expression of HER2 has not been tested in patients because this study was initiated before the publication of ToGA trial. Results of the current study may only be restricted to AGC patients without over-expressed HER2. Therefore, the results of our subgroup analyses need to be validated in larger clinical trials with the consideration of HER2 expression. Further studies are needed to evaluate the extrapolation of results from this trial to real-world settings.
In conclusion, the PACX regimen did not improve the PFS and OS compared with the XP regimen in patients with AGC. But, patients receiving PACX showed significantly higher ORR, improved QoL, and reduced incidences of AEs such as blood and lymphatic system disorder and gastrointestinal disorders than those receiving XP. Thus, PACX is an effective treatment with milder toxicities, but so far it may not be able to replace the first-line standard chemotherapy of XP. Future research of biomarkers for improving efficacy in identifying highly responsive patients is warranted.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–8.
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:57–63.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Sakamoto J, Matsui T, Kodera Y. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer. 2009;12:69–78.
Sakamoto J, Chin K, Kondo K, Kojima H, Terashima M, Yamamura Y, et al. Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs. 2006;17:231–6.
Ryu MH, Kang YK. ML17032 trial: capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in advanced gastric cancer. Expert Rev Anticancer Ther. 2009;9:1745–51.
Koizumi W, Saigenji K, Ujiie S, Terashima M, Sakata Y, Taguchi T, et al. A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology. 2003;64:232–6.
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73.
Park YH, Ryoo BY, Choi SJ, Kim HT. A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer. 2004;90:1329–33.
Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer–a GERCOR study. J Clin Oncol. 2006;24:394–400.
Gong J, Hu B, Zhang X, Zhang F, Zhang J, Xu N, et al. The multicenter, phase II prospective study of paclitaxel plus capecitabine as first-line chemotherapy in advanced gastric carcinoma. Oncologist. 2014;19:173–4.
Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer. 2008;98:316–22.
Yuan M, Yang Y, Lv W, Song Z, Zhong H. Paclitaxel combined with capecitabine as first-line chemotherapy for advanced or recurrent gastric cancer. Oncol Lett. 2014;8:351–4.
Kano Y, Akutsu M, Tsunoda S, Ando J, Matsui J, Suzuki K, et al. Schedule-dependent interaction between paclitaxel and 5-fluorouracil in human carcinoma cell lines in vitro. Br J Cancer. 1996;74:704–10.
Ishitsuka H. Capecitabine: preclinical pharmacology studies. Invest New Drugs. 2000;18:343–54.
Ajani J. Review of capecitabine as oral treatment of gastric, gastroesophageal, and esophageal cancers. Cancer. 2006;107:221–31.
Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291–7.
Acknowledgements
We also thank Dr Guanghai Dai from PLA General Hospital, Dr Jifeng Feng from Jiangsu Cancer Hospital, Dr Chunmei Bai from Peking Union Medical College Hospital, Dr Jun Zhang from Ruijin Hospital, Dr Nanfeng Fan from Fujian Provincial Cancer Hospital, and Dr Lihong Cui from Navy General Hospital for their contribution to the study. This work was supported by the National Key Research and Development Program of China (No. 2017YFC1308900, 2017YFC0908400), Beijing Municipal Administration of Hospital Clinical Medicine Development of Special Funding Support (No. Z161100002616036, Z141107002514013), Beijing Municipal Science & Technology Commission Program (No. Z161100002616036, Z141107002514013), Beijing Natural Science Foundation (7161002), and Capital’s Funds for Health Improvement and Research (2016-1-1021).
Author information
Authors and Affiliations
Contributions
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. As the leading principle investigator of the study, Professor Lin Shen contributed significantly to study design, data analysis, interpretation of the data, critical evaluation and clinical management; all authors were involved in developing the original study and protocols. Xiaotian Zhang contributed to data analysis and drafting the manuscript. Zhihao Lu and Lin Shen have also contributed to drafting the manuscript. Zhihao Lu, Wei Liu, Tianshu Liu, Xiaotian Zhang, Bing Hu, Wei Li, Qingxia Fan, Jianming Xu, Nong Xu, Yuxian Bai, Yueyin Pan, Qing Xu, Wei Bai, Li Xia, Yong Gao, Wenling Wang, Yongqian Shu and Lin Shen were responsible for the patient enrolment. All authors provided significant input to the paper by means of critical revisions and have read and approved the final manuscript. Medical writing services were provided by Cactus Communications and paid for by Shanghai Roche Pharmaceuticals Ltd., China. The authors retained full control of manuscript content.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest. This work was supported by Shanghai Roche Pharmaceuticals Ltd., China and Haiyao Ltd. This study is an investigator initiated study. Roche Ltd. provided the drug capecitabine and part of funding. Haiyao Ltd. provided part of paclitaxel. These two corporations played no role in study design, data collection, data analysis, data interpretation.
Ethical standards
The study protocol was approved by the institutional review board of Peking University Cancer Hospital and respective independent ethics committees at each investigating site. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
All participants included in the study provided written informed consent.
Additional information
Zhihao Lu, Xiaotian Zhang, Wei Liu and Tianshu Liu contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lu, Z., Zhang, X., Liu, W. et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer 21, 782–791 (2018). https://doi.org/10.1007/s10120-018-0809-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-018-0809-y