Abstract
Purpose
Conventional approaches for enhancing wound healing may not always yield satisfactory results. Instead, we test the effectiveness of a newly developed photodynamic therapy (PDT) that uses methylene blue (MB) loaded with polyethylene glycol (PEG) (MB-PEG) hydrogel to accelerate wound healing process in mice.
Methods
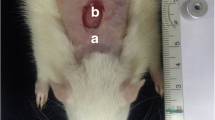
A dorsal skin incision with 6 mm punch which topically subjected to MB-PEG hydrogel and a low-level laser light of red light to assess the regeneration process of wounded skin. A total of 63 adult male CD1 mice divided into normal group (no treatment) and other wound groups received different treatments of laser (650 ± 5 nm and power intensity of 180 mW/cm2), MB-PEG, or PDT (MB-PEG followed by laser). The wound healing parameters were investigated by histological examination of the skin and measuring of proinflammatory cytokines at the early stage (48 h) and a late one on day 21. Results: at 48 h, the score of tissue granulation, inflammation, and angiogenesis process were markedly improved in wounded groups that received MB + PEG combined with laser compared to the group treated with laser alone. On day 21, a significant improvement of the inflammation was detected in the group treated with MB + PEG plus laser compared to the other groups. At 48 h, the upregulated serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-1β in the wound group were significantly (P < 0.001) reduced in the group treated with MB + PEG combined with laser.
Conclusion
MB-PEG based hydrogel improves and accelerates wound closure in the context of laser compared to either single treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A skin wound results from the breakdown of the epidermal layer integrity. The wound healing process has four overlapping phases which are hemostasis, inflammation, proliferation, and remodeling [1]. Pre-clinical research on wound healing is a hot topic, especially when it comes to factors that boost healing speed or quality.
Photodynamic therapy (PDT) is one of the most brilliant therapies that used to treat malignancies, infectious disorders, and inflammatory diseases. In this type of therapy, a photosensitizer (PS) is delivered either systemically, regionally, or topically, to a patient with a lesion, followed by lighting of the lesion with visible light, which generates cytotoxic species in the presence of oxygen, resulting in cell death and tissue damage. The PDT impacts the production of inflammatory mediators such as cytokines, growth factors, and proteins [2]. The role of Low-level laser therapy (LLLT) comes to promote lymphocyte proliferation, increase fibroblast’s secretion of growth factors and enhance the uptake of both fibrin and collagen [3].
In topical PDT, the wound site should receive a sufficient concentration of PS; however, retention of liquid PS application is problematic [4]. In this approach, a fresh formulation of PS-loaded hydrogel is being researched in order to get around this issue. Many studies have attempted to address the problem of PS’s solubility; curcumin-Pluronic® F-127; [5]. Nevertheless, another research has looked into ways to improve the delivery of PS to the intended wound site; films containing chlorin p6 (Cp6, anionic PS) or methylene blue (MB), cationic PS) were prepared using sodium alginate (SA), pectin (PC), and carboxymethyl cellulose (CMC) [6]. However, a number of studies have shown that using polyethylene glycol (PEG)–protein wound dressings will enhance their hydrating action and hasten the healing process [7].
In terms of controlling the inflammatory response, PDT functions by suppressing the expression of nuclear factor-kappa B (NF-kB) and proinflammatory interleukins (IL) such as IL–1α, IL-1β, and IL-2, as well as tumor necrosis factor-α [8]. However, poly-L-lysine-conjugated chlorin p6 (pl-cp6) mediated PDT improves angiogenesis in diabetic rats [2].
Among the phenothiazine dyes, MB exhibits photosensitization with light absorption at 660 nm. It also has a benign nonallergic impact and effective against a variety of pathogens, such as viruses, fungus, and bacteria [9]. Moreover, in MB-PDT, the innate immune response against infection is mainly supported by polymorphonuclear neutrophils (PMN) that, once recruited to the infected site, it ingests and kill microbes [10], Thus, it plays an irreplaceable role in limiting infections, extending the lifespan of skin fibroblasts and enhancing cell proliferation [11].
The nanoparticles (NPs) are utilized in the delivery of non-water-soluble PSs more rapidly to the wounded part thus accelerating wound healing. The PEG is synthetic polymer that is non-toxic, inert, and appropriate for usage in medical devices [12]. The goal of this work was to create a hydrogel based on MB-PEG conjugates that could be topically given to mice with incisional skin lesions to hasten the healing process. Therefore, PEG plays a crucial role in creating a flexible synthetic hydrogel in the form of NPs. It also reduces inflammation at the onset of wound healing by modulating tissue-biomaterial interactions, which is advantageous for accelerated tissue repair and excellent cosmetic outcomes [7]. Also, the hydrogel’s efficiency was compared to each single treatment. According to our knowledge, the combination treatment of PDT with MB-PEG hydrogel might help mice’s wounds heal faster by initiating early anti-inflammatory responses.
Materials and methods
Hydrogel preparation (MB-loaded PEG)
According to reference [13], 40 mL of anhydrous 4-chlorophthalonitrile (Sigma-Aldrich) was mixed with 3.08 g of MB (equivalent to 5 mmol) and 2.6 g of PEG 800 (equivalent to 7.5 mmol). Subsequently, 4 g (29 mmol) of anhydrous K2CO3 were added. After being mixed for 24 h at a temperature of 65 °C, the reaction mixture was filtered and then diluted with dichloromethane (Sigma-Aldrich). The diluted mixture was then extracted using distilled water. In order to obtain the PEG-conjugated MB, the organic layer was dehydrated using Na2SO4 and concentrated. To further purify the solution, 200 mg or 1.5 mmol of zinc chloride were introduced after dissolving PEG-linked MB in a solution containing 10 mL of dimethylaminoethanol and 5 mL of n-butanol. The reaction mixture was stirred at a temperature of 100 °C for a duration of 24 h, while being exposed to nitrogen pressure. The solid returned to its original form after cooling.
Photodynamic therapy
Mice were exposed to a diode laser (LSR-PS-ll#10,042,504 - Germany) with emitted a red laser light of wavelength 650 ± 5 nm, power intensity of 180 mW/cm2 (Table 1& Fig. 1S) and spot radius 6 mm at a distance of 20 cm from the injured skin part for 5 min [14]. Firstly, injured mice were treated topically with MB-loaded PEG (hydrogel) and kept in the dark for an hour to prevent MB photoexcitation and poisoning before exposure to laser (Fig. 2S).
Photomicrograph of wounded areas of different mice groups at 48 h post-wounding (4X, scale bar = 100µm; 10X, scale bar = 50µm). All groups showed marked acute severe inflammation occupying the wound gap with serofibrinous exudates and necrotic crusts covering. (Cr) crust, (Ed) edema, (H) hemorrhages, (N) necrosis. Charts showing histopathological parameters of wound healing evaluation at 48 h. Data are expressed as a mean ± SEM. Significant difference was considered at P < 0.05. PEG group: mice treated with MB-PEG, PEG+L group: mice treated with MB-PEG+L
Ethical consideration
A total of sixty-three male albino mice, with an average weight of 22 ± 5 g, were given normal diet pellets and had access to water ad libitum. They were housed in standard cages. The mice were maintained at a temperature of 22 ± 3 °C with a 12-h light/dark cycle. They were given a period of 7 days to acclimate before the experiment began [15].
Wounding skin incision strategy
All animal groups despite the normal group were shaved at their back by an electric animal shaver to facilitate wounding [16], after that, each animal was partially numbed with isoflurane anesthetic solution (Pharco - Egypt) for a few seconds then with a punch biopsy tool (Indiamart Bb - India), a circular wound with a diameter of 6 mm was performed on each animal shaved back.
Experimental design
A total of sixty-three mice were employed for this study, dividing them into five groups of fourteen mice each. After 48 h of wound induction, half of each group was euthanized, while the other half lived for 21 days. Group N: normal mice. Group W: injured mice receiving no treatment. Group L: injured mice were exposed immediately with laser light after wound induction and conducted three times a week for 21 days [17] (Fig. 2S). Group MB-PEG: injured mice treated immediately by topical administration of MB-PEG hydrogel after wound induction and conducted three times a week for 21 days. Group MB-PEG + L: injured mice treated immediately by combining a topical administration of MB-PEG hydrogel and exposure to laser after wound induction, conducted three times a week for 21 days. The diameter of the wound was measured by a caliper and recorded twice a week. The wounded area was capped during the experimental period of 21 days on days 3, 7, 11, 15, 18 and 21 as well as wound closures were compared with the original measurements (wound healing rate). The healing rate was estimated as the following calculation:
% = area before treatment - wound area after treatment / wound area before treatment.
The data are expressed as a percentage of early healing rate [18]. The animals were subjected to mild anesthesia using isoflurane [19]. Blood samples were then obtained from the venous orbital plexus of mice. Subsequently, the animals were euthanized through cervical dislocation [20].
Histological examination
Prior to cervical dislocation, the animals were rendered unconscious through the inhalation of isoflurane [20]. The wound skin was gathered and preserved in a solution of 10% neutral buffered formalin. Washing was done in tap water then serial dilutions of alcohol (ehanol) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 degrees in hot air oven for 24 h. Paraplast wax tissue blocks were prepared for sectioning at 4 microns thickness by rotatory microtome. The obtained tissue sections were collected on glass slides, deparaffinized, stained by hematoxylin & eosin stain [21], then slide sections were examined through the Olympus BX43 light microscope and photographed using the Cellsens dimensions software (Olympus) linked to Olympus DP27 camera.
Differential blood count
Complete blood picture is performed using an automated hematology analyzer, which counts red blood cells, white blood cells (WBCs) and platelets. The concentration of hemoglobin (HG) was measured, and the red blood cell indices are calculated from the red blood cell count, average red cell volume, and HG [22].
Cytokines assays
Quantitative determination of tumor necrosis factor (TNF)-α and interleukin (IL)-β levels [23] was performed using a sandwich enzyme-linked immunosorbent assay (ELISA) according to manufactured instructions (Glory Science Co., Ltd). Optical density was measured by a plate reader (Das, Italy) at 450 nm wavelength.
Statistical analysis
The Statistical Package for the Social Sciences (IBM-SPSS, v.26) was used for all statistical analyses. The effect of experimental time (48 h and 21 days) on the studied parameters was applied by using a one-way analysis of variance (ANOVA). Post comparison and Duncan Multiple Range Test (DMRT) were used to detect significant differences in the intervals of the wounded groups. For all tests, data were represented as a mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
Results
Healing rate
The entire wound healing process was assessed on days 3, 7, 11, 15, 18 post-wounding. Images were captured to measure the wound areas and the border of each wound was traced on an image to measure the healed area (Fig. 1). The wound healing rate was significantly affected by different time points (P < 0.01). The wound healing rate was remarkably improved as a result of treating wounded mice with laser, MB-PEG, and MB-PEG + laser, however, group that received treatment with MB-PEG + laser showed the most effective one with a significant (P < 0.05/) increase in wound closure, the wound areas were entirely covered by the epidermis and the color of the wounds were close to normal skin, whereas the impact of treatment with laser alone was incomplete, and delayed till the end of experiment. Along 21days post-wounding, the healing rate (%) was significantly affected by the different time points, however, single treatment of MB-PEG or admixed with laser significantly increased the rate (%) of wound healing revealing 100% at day 21 compared to the wound group or laser group with a healing ratio of 93.28% and 98.42% respectively (Table 2).
Effects of PDT on histological architecture of skin wounds staining
Examination of 48 h post-induction of the wound was investigated in all groups. The early wound healing process was comparable in all experimental groups. The wound surface was covered by a thick crust with an underlying intense inflammatory reaction that was composed of acute inflammatory cells infiltration mainly neutrophils, edema, hemorrhage, and necrotic tissue in most circumstances. Regarding the re-epithelization score, although the application of (MB-PEG) showed early epithelial proliferation that started at the wound edges either alone or admixed with laser, no significant difference was detected between all groups. The granulation tissue and the inflammation scores showed marked a significant improvement in MB-PEG + L compared to groups W or L. The angiogenesis process showed a marked significant enhancement in groups receiving MB-PEG in comparison with other groups (Fig. 2).
On day 21, group W showed poor wound healing that was characterized by incomplete epidermal remodeling with the persistence of necrotic serofibrinous crust. The wound gap was occupied by inflammatory granulation tissue which revealed excessive mononuclear inflammatory cells infiltration, necrotic debris, and aggregations of bacterial colonies. Complete wound closure was observed in group L, group MB-PEG and group MB-PEG + L which showed a newly formed epidermal covering layer and evidence of keratinization in some instances accompanied by varying grades of filling granulation tissue which showed a variable number of inflammatory cells, collagen bundles, and newly formed blood vessels. The statistical analysis showed a marked significant reduction in re-epithelization and granulation in all parameters in group L, compared to the other treated groups. Concerning re-epithelization, granulation tissue, and angiogenesis scores, the absence of significant difference was detected in the group receiving MB-PEG compared to MB-PEG + laser group. Meanwhile, a significant improvement in inflammation was detected in group MB-PEG + L compared to the other groups. The application of MB-PEG admixed with laser revealed the highest wound healing with healthy epidermal covering and organized granulation tissue (Fig. 3).
Photomicrograph of wounded areas of different mice groups after 21 days post-wounding (4x, scale bar 100 μm; 10x, scale bar 50 μm). Group W showing improper wound healing with absence of epidermal covering. Groups L, PEG and PEG + L showing compete epidermal growth and decrease the inflammation of the formed granulation tissue. Keratinization is presenting in PEG treated groups. (E) Epidermal layer, (G) granulation tissue, (I) inflammation, (N) necrosis. Charts showing parameters of wound healing evaluation at day 21. Data Expressed as means ± SEM. Significant difference was considered at P < 0.05. PEG group: mice treated with MB-PEG, PEG + L group: mice treated with MB-PEG + L
Differential blood count
After 48 h of wound induction, the wound group revealed significantly lower values of HB% and monocytes count compared to the normal group. Treatment with laser resulted in a significant increase in the count of platelets, lymphocytes, and monocytes while group MB+PEG exhibited significantly higher values of HB% and monocytes and lower values of lymphocytes count compared to the wound group. The combination treatment of MB-PEG plus laser significantly caused an elevation in values of HB%, total count of WBC, lymphocytes, and monocytes aw well as lowered values of neutrophils compared to the wound group (Table 3).
On day 21, non-significant differences were observed regarding all measured parameters in the wound group compared to the normal group. The wounded mice treated with either laser or MB-PEG showed a significant decrease in the Hb% as well as a significant increase in the count of platelets and neutrophils compared to the wound group. Combination treatment of of MB-PEG plus laser resulted in significantly higher values of platelets and neutrophils while significantly lower counts of WBCs, lymphocytes, and monocytes compared to the wound group (Table 4).
Cytokines profile
A Comparison of the proinflammatory cytokines TNF-α and IL1β serum levels was investigated after 48 h and 21 days among experimental groups. At 48 h of wound injury, the serum levels of TNF-α were significantly increased (P < 0.001) in the wounded group compared to the normal group, while direct medication of the other groups with the action of laser, MB-PEG, or MB-PEG + laser caused a significant (P < 0.05, P < 0.01, and P < 0.001, respectively) decrease of TNF-α levels compared to the wounded untreated group. At the end of the study on day21, no significant differences were detected between the wounded group and the other groups with various treatments (Table 5).
During the first 48 h, the IL-1β serum levels in the wounded group showed significantly higher levels (P < 0.001) than those of the normal group while the wounded groups treated with laser, MB-PEG, or MB- PEG + laser exhibited significantly lower levels (P < 0.05, P < 0.01, and P < 0.001) respectively than those wounded group. On the other hand, at the end of the study, IL-1β still showed significantly higher levels (P < 0.001) in the wounded group compared to the normal group but all other groups that received various treatments showed no significant differences compared to the wounded group (Table 6).
Discussion
Healing process following tissue injury includes homeostasis, inflammation, proliferation, and tissue remodeling phases [24]. Achieving the fastest healing rate and reepithelization is the aim of this investigation. In topical PDT, a crucial stage in treating wound site is delivering PS at the right concentration. As a result, PS must be applied in the right formulation at the affected site [6]. The recently developed MB-PEG hydrogel outperformed all expectations in terms of its ability to promote wound healing and reepithelialization. The role of MB comes to reduce inflammation and protect against free radicals, thus, it hastens the healing process [25], while the PEG-based hydrogel showed good biocompatibility and safety [26]. After applying the MB-PEG hydrogels, bleeding from the incisions on the dorsum of mice immediately stopped, and the wound openings closed within few min while the wound openings did not close after applying laser only. Along 21 day, both groups that treated with MB-PEG hydrogel and MB-PEG + laser recorded highest percent of wound healing rate and the wounds have a distinct appearance from a macroscopical and histological perspective. The impact of PEG-based chitosan (hg-PEGDA-Q) was also thoroughly examined by [27] who suggested that hydrogel scaffolding networks could be a therapeutic substitute to quicken the healing process in mice under both normal and diabetic settings. Also, according to [28], the polyethylene glycol/triethoxysilane-modified polyurethane (PUESi) dressing improved wound healing in rats by generating micronegative pressure through its high absorption capacity with deformation, in addition to being made using an easy-to-use and effective technique.
Histologically, 48 h post-wounding; the inflammation score of infiltrated neutrophils, edema, hemorrhages and necrotic tissue was significantly elevated in the groups treated with laser, MB-PEG hydrogel or MB-PEG + laser compared to wounded group, During the inflammation phase, the granulation tissue is primarily composed of predominant inflammatory cells, mainly neutrophils that are recruited to the wound site and removed during the repair process. However, during the proliferative phase, endothelial cells, macrophages, and fibroblasts begin to fill the wound area to restore tissue integrity [24]. Twenty-one days post-wounding, the best rate of wound healing with a healthy epidermal layer and well-organized granulation tissue was demonstrated by the group treated with MB + PEG hydrogel or admixed with laser. Several studies approved that PDT improves angiogenesis and tissue healing, including regular epithelial lining, decreased fibrinous exudate, and more organized and thick conjunctive tissue, which resulted in full re-epithelization and keratin production [2, 29,30,31]. The outcomes of this investigation were corroborated with [32] who concluded that topical application of low dose Foslip® in a collagen matrix followed by illumination considerably accelerates wound healing.
Among the hematological parameters, the HG% was significantly decreased either in laser treated group or MB-PEG + laser group compared the wounded and normal group at late phase of recovery (day21). A previous study denoted to the decrease in the erythrocytes volume by the exposure to LLLT [33]. This is because LLLT can split cell membranes, increasing porosity and attracting calcium ions that are free in extracellular solution and move to the intracellular fluid of the erythrocytes. Thus, this rise causes the passage of K + ions into the extracellular fluid, which causes a decrease in the MCV of erythrocytes [34]. White blood cells are key players in inflammation because they operate as phagocytes in the tissue, removing bacteria and cellular debris. After 48 h of injury, the total count of WBCs of wounded mice treated with both laser and MB-PEG hydrogel significantly promoted higher counts of WBCs while at the end of study by day 21 counteract the increase of the count compared to wounded or normal group. These findings may denote to the effect of photodynamic therapy in stimulation of WBCs production in early phases while after a long period of photodynamic therapy has inverse effect by decreasing the WBCs count. In a previous study, an increase in leukocytes was linked to the elevation in mitochondrial intracellular ATP [35]. Neutrophils play a crucial role in the early stages of inflammation by helping to restore hemostasis, perform phagocytosis, and release extracellular chemical messengers. They are attracted to wound sites in huge numbers, where they release cytokines and toxic substances that foster an inflammatory environment. Inflammation reaches its peak 48 h after injury, and during this period, the site of injury begins to malfunction and become red, hot, swollen, and painful. Differential count of neutrophils showed significant higher levels in wounded mice compared to the normal group after 48 h of injury. These findings are comparable with [36] and [37] who showed that in full-thickness incision wounds in mice displayed infiltration of the tissue by neutrophils and macrophages for at least 13 days. Whereas application of MB-PEG or MB-PEG + laser along 21 days significantly induced a rise in the count of neutrophils compared to wounded and normal groups, these results are compatible with [10] who found that in MB mediated PDT; human neutrophils adhesion increases and does not modify myeloperoxidase release. Furthermore, application of MB-PEG+laser promoted a significant reduction in the count of lymphocytes compared to wounded group. These findings are consistent with [38], who discovered that the mitogen phytohemagglutinin significantly suppressed lymphocyte proliferation after incubating lymphocytes for seven days.
In the context of PEG-functionalized NPs, polymeric forms with dimensions of less than 31.5 nm could effectively avoid immune cells, while those larger than 50.2 nm triggered anti-PEG [39]. In this study, the dimension of PEG NPS used ranged from 25 to 30 nm which in turn facilitates penetration through the membrane and improves the distribution of the ROS, hence improving the efficacy of anti-inflammation PDT.
Regarding to the levels of the proinflammatory cytokines, the study’s findings confirmed the hypothesis by showing that 48 h-post wounding, whereas MB-PEG hydrogel when topically applied to incisional wounds individually or admixed with laser, it can diminish the levels of TNF-α and IL-1β in vivo. This sequestration action causes a markedly decreased input of immune cells influx into the wound [40]. The decreased inflammatory signaling pathway enhances the wound environment, which in turn stimulates vascularization, re-epithelialization, and granulation tissue development. The approach using PEG in inflammatory lung injury in humans has been reported by [41]. They found that PEG has the ability to combine many endothelial cell barrier-regulatory chemicals to quickly activate signal transduction pathways that enhance barrier function and target the cytoskeleton. In addition, MB has long been known for its anti-inflammatory qualities [9]. The capacity of MB in decreasing both STAT3 activation and the serum levels of IL-6 in LPS-administered mice have suggested important mechanisms underlying the effects of MB on inflammation [42].
Low level laser therapy (LLLT) has been shown to be successful in treating inflammation in a number of investigations, and these studies also show that laser light has the ability to modulate pro- and anti-inflammatory mediators [43,44,45,&46]. Groups that were administered with LLLT 48 h after injury showed a statistically significant decrease in the expression of IL-1β and TNF-α levels protein when compared with the non-treated wound group, but 21 days after injury the statistical analysis returned to levels similar to that of the normal group [47]. It has been suggested that LLLT with 50 mW was efficient in modulating inflammatory mediators (IL-1β and IL-6) and inflammatory cells (macrophages and neutrophils), which correlated with the histology that showed a reduction in the inflammatory process. However, in this investigation, the outcomes obtained from treating with MB-PEG hydrogel were superior to those obtained from treating with LLLT, both histologically and physiologically, as a result, the inflammatory phase and the remodeling phase begin earlier in MB-PEG based hydrogels treatment.
Conclusion
Based on the data collected, it appears that MB-PEG hydrogel facilitates the healing process of acute wound damage. When LLLT is applied in combination with MB-PEG hydrogel, the healing rate is increased and the skin’s morphological and histological characteristics are improved. However, the effects of LLLT alone on inflammation and wound closure are somewhat less favorable.
References
Broughton G, Janis JE, Attinger CE (2006) The basic science of wound healing. Plast Reconstr Surg 117(7 Suppl):12S–34S
Sahu K, Sharma M, Dube A, Gupta PK (2015) Topical antimicrobial photodynamic therapy improves angiogenesis in wounds of diabetic mice. Laser Med Sci 30(7):1923–1929
Channual J, Choi B, Osann K, Pattanachinda D, Lotfi J, Kelly KM (2008) Vascular effects of photodynamic and pulsed dye laser therapy protocols. Lasers Surg Med. 2008;40(9);644 – 50
Cassidy CM, Tunney MM, McCarron PA, Donnelly RF (2009) Drug delivery strategies for photodynamic antimicrobial chemotherapy: from bench top to clinical practice. J Photochem Photobiol B 95(2):71–80
Dos Santos DDL, Besegato JF, de Melo PBG, Junior JAO, Chorilli M, Deng D, Bagnato VS, de Souza Rastelli AN (2022) Effect of curcumin-encapsulated Pluronic® F-127 over duo-species biofilm of Streptococcus mutans and Candida albicans. Lasers Med Sci. 2022;37(3):1775–1786. https://doi.org/10.1007/s10103-021-03432-9. Epub 2021 Oct 19. PMID: 34664132
Sharma M, Dube A, Majumder SK (2021) Antibacterial photodynamic activity of photosensitizer-embedded alginate-pectin-carboxymethyl cellulose composite biopolymer films. Lasers Med Sci. 2021;36(4):763–772. https://doi.org/10.1007/s10103-020-03083-2. Epub 2020 Aug 7. PMID: 32767164
Shingel KI, Di Stabile L, Marty JP, Faure MP (2006) Inflammatory inert poly(ethylene glycol)--protein wound dressing improves healing responses in partial- and full-thickness wounds. Int Wound J. 2006;3(4):332 – 42. https://doi.org/10.1111/j.1742-481X.2006.00262.x. PMID: 17199768; PMCID: PMC7951209
Sahu K, Sharma M, Gupta PK (2015) Modulation of inflammatory response of wounds by antimicrobial photodynamic therapy. Laser Ther. 2015;24(3):201-8. https://doi.org/10.5978/islsm.15-OR-13. PMID: 26557735; PMCID: PMC4639678
Hirakawa K, Mori M (2021) Phenothiazine dyes induce NADH Photooxidation through Electron transfer: kinetics and the effect of copper ions. ACS Omega 6(12):8630–8636. https://doi.org/10.1021/acsomega.1c00484PMID: 33817524; PMCID: PMC8015084
Trevisan E, Menegazzi R, Zabucchi G, Troian B, Prato S, Vita F, Rapozzi V, Grandolfo M, Borelli V (2019) Effect of methylene blue photodynamic therapy on human neutrophil functional responses. J Photochem Photobiol B. 2019;199:111605. https://doi.org/10.1016/j.jphotobiol.2019.111605. Epub 2019 Aug 25. PMID: 31473428
De Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AO (2006) Photosensitization of different Candida species by low power laser light. J Photochem Photobiol 83:34–38. https://doi.org/10.1016/j.jphotobiol.2005.12.002
Yesilirmak N, Diakonis VF, Battle JF, Yoo SH (2015) Application of a hydrogel ocular sealant to avoid recurrence of epithelial ingrowth after LASIK enhancement. J Refract Surg 31(4):275–277
Feng Lv B, Cao Y, Cui, Liu T (2012) Zinc Phthalocyanine Labelled Polyethylene Glycol: Preparation, characterization, Interaction with bovine serum albumin and Near Infrared fluorescence imaging in vivo. Molecules 17:6348–6361. https://doi.org/10.3390/molecules17066348
Ahmed OF (2007) Laser photodynamic therapy for Ehrlich solid tumours using methylene blue photosensitizer (experimental study), PhD thesis, NILES, Cairo University, http://srv3.eulc.eg/eulc_v5/libraries
Parasuraman S (2022) Care and Handling of Laboratory animals. In: Lakshmanan M, Shewade DG, Raj GM (eds) Introduction to basics of Pharmacology and Toxicology. Springer, Singapore. https://doi.org/10.1007/978-981-19-5343-9_3.
Dwivedi D, Dwivedi M, Malviya S, Singh V (2017) Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in Wistar rats. J Traditional Complement Med 7(1):79–85. https://doi.org/10.1016/j.jtcme.2015.12.002
Nussbaum EL, Heras FL, Pritzker KP, Mazzulli T, Lilge L (2014) Effects of low intensity laser irradiation during healing of infected skin wounds in the rat. Photonics Lasers Med. 2014;3(1):23–36. https://doi.org/10.1515/plm-2013-0049. PMID: 26225295; PMCID: PMC4516410
Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, Frade MAC (2020) Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. https://doi.org/10.1111/iep.12346. Epub 2020 Mar 30. PMID: 32227524; PMCID: PMC7306904
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. Jul;1(2):87–93. https://doi.org/10.4103/0976-500X.72350. Erratum in: J Pharmacol Pharmacother. 2017 Jul-Sep;8(3):153. PMID: 21350616; PMCID: PMC3043327
Sharma A, Fish BL, Moulder JE, Medhora M, Baker JE, Mader M, Cohen EP (2014) Safety and blood sample volume and quality of a refined retro-orbital bleeding technique in rats using a lateral approach. Lab Anim (NY) 43(2):63–66. https://doi.org/10.1038/laban.432PMID: 24451361; PMCID: PMC3989930
Bancroft JD, Suvarna SK, Layton C Bancroft’s Theory and Practice of Histological Techniques, Expert Consult: Online and Print, 7: Bancroft’s Theory and Practice of Histological Techniques. Elsevier Health Sciences.Bancroft’s Theory and Practice of Histological Techniques,8th Edition - February 27, 2018,Authors: Kim S Suvarna, Layton C (2013) John D. Bancroft,Language: English,Hardback ISBN: 97807020686459 7 8-0-7 0 2 0–6 8 6 4–5 eBook ISBN: 9780702068867
Celkan TT (2020) What does a hemogram say to us? Turk Pediatri Ars. Jun 19;55(2):103–116. https://doi.org/10.14744/TurkPediatriArs.2019.76301. PMID: 32684755; PMCID: PMC7344121
Kara YA (2018) The Measurement of Serum Tumor Necrosis Factor-alpha Levels in Patients with Lichen Planus. Indian J Dermatol. Jul-Aug;63(4):297–300. https://doi.org/10.4103/ijd.IJD_474_17. Retraction in: Indian J Dermatol. 2019 May-Jun;64(3):165. PMID: 30078872; PMCID: PMC6052759
Eming SA, Krieg T, Davidson JM (2007) Inflammation in wound repair: molecular and cellular mechanisms. J Investig Dermatol Symp Proc 127(3):514–525
Rosique MJ, Rosique RG, Faria FM, Oliveira CC, Farina JA Jr, Évora PR (2017) Methylene blue reduces progression of burn and increases skin survival in an experimental rat model. Burns 43(8):1702–1708
Ho VY, Shah GK, Liu EM (2015) ReSure sealant for pars plana vitrectomy wound closure. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1042–1044
García AEB, Merino MVF, Tolosa IMG, Muñoz DR, López MAC, Pantoja RO (2020) Polyethylene-glycol chitosan hydrogel accelerates traumatic wound healing in diabetic mice. FASEB J 34:1–1. https://doi.org/10.1096/fasebj.2020.34.s1.04577
Chen CF, Chen SH, Chen RF, Liu KF, Kuo YR, Wang CK, Lee TM, Wang YHA (2023) Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing. Int J Mol Sci. 2023;24(15):12506. https://doi.org/10.3390/ijms241512506. PMID: 37569881; PMCID: PMC10419382
Simonetti O, Orlando F, Alongi C, Lucarini G, Silvestri C, Zizzi A, Fantetti I (2011) Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068⁄Cl in an experimental model of Staphylococcus aureus wound infection. Br J Dermatol 164:987–995
Cesar GB, Winyk AP, Sluchensci Dos Santos F, Queiroz EF, Soares KCN, Caetano W, Tominaga TT (2022) Treatment of chronic wounds with methylene blue photodynamic therapy: a case report. Photodiagnosis Photodyn Ther 39:103016. https://doi.org/10.1016/j.pdpdt.2022.103016Epub 2022 Jul 14. PMID: 35840009
Shen X, Dong L, He X, Zhao C, Zhang W, Li X, Lu Y (2020) Treatment of infected wounds with methylene blue photodynamic therapy: an effective and safe treatment method. Photodiagnosis Photodyn Ther 32:102051 Epub,Oct 12. PMID: 33059110
Garrier J, Bezdetnaya L, Barlier C, Grafe S, Guillemin F, Hallewin MA (2011) Foslip®-based photodynamic therapy as a means to improve wound healing. Photodiagnosis Photodyn Ther 8:321–327
Yu JB, Jairam V, Lee V, Park HS, Thomas CR, Melnick ER, Adelson KB (2019) Treatment related complications of systemic therapy and Radiotherapy. JAMA Onco 5(7):1028–1035. https://doi.org/10.1001/jamaoncol.2019.0086
de Oliveira MC, Krueger GF, Sganzerla JT, Gassen HT, Hernández PAG, Allgayer MDC, Miguens-Jr SAQ (2021) Effect of Radiotherapy and low-level laser therapy on circulating blood cells of rats. J Lasers Med Sci 12:e45. https://doi.org/10.34172/jlms.2021.45PMID: 34733768; PMCID: PMC8558724
Al Musawi MSA, Jafar MS, Al-Gailani BT, Ahmed NM, Suhaimi FM, Suardi N (2016) In Vitro Mean Red Blood Cell volume Change Induced by Diode Pump Solid State Low- Level laser of 405 nm. Photomed Laser Surg 34(5):211–214
Šitum K, Bokulić A, Ivetić-Tkalčević V, Parnham MJ, Čužić S, Đurić K et al (2007) Comparison of systemic inflammatory and hematology parameters in normal C57Bl/6 and genetically diabetic db/db mice during local wound repair. Biochem Med (Zagreb 17:85–93
Lofreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ (1998) Leptin regulates proinflammatory immune responses. FASEB J 12:57–65
Zhang B, Cheng Z, Mo Q, Wang L, Wang X, Wu X, Jia Y, Huang Y (2015) Functional inactivation of lymphocytes by methylene blue with visible light. Photochem Photobiol Sci 14(10):1903–1909. https://doi.org/10.1039/c5pp00220f. PMID: 26295729
Abu Lila AS, Kiwada H, Ishida T (2013) The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release 172:38–47
Nadine, Lohmann et al (2017) Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med.9,eaai9044(2017).https://doi.org/10.1126/scitranslmed.aai9044
Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC, Garcia JG (2009) Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res 77:174–186
Li Y, Ying W (2023) Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Front Immunol 14:1181932. https://doi.org/10.3389/fimmu.2023.1181932PMID: 37325623; PMCID: PMC10266349
Bashardoust ST, Macdermid JC, Houghton P, Grewal R (2010) Effects of low power laser irradiation on bone healing in animals: a meta-analysis. J Orthop Surg Res 5:1–13
Laraia EM, Silva IS, Pereira DM, Dos Reis FA, Albertini R, de Almeida P, Leal Junior EC, de Tarso P (2012) Effect of low-level laser therapy (660 nm) on acute inflammation induced by tenotomy of Achilles tendon in rats. Photochem Photobiol. 2012;15:1546–1550. https://doi.org/10.1111/j.1751-1097.2012.01179.x
de Lima FM, Bjordal JM, Albertini R, Santos FV, Aimbire F (2010) Low-level laser therapy (LLLT) attenuates RhoA mRNA expression in the rat bronchi smooth muscle exposed to tumor necrosis factor-alpha. Lasers Med Sci. 2010;15:661–668. https://doi.org/10.1007/s10103-010-0766-0
Pallotta RC, Bjordal JM, Frigo L, Leal Junior EC, Teixeira S, Marcos RL, Ramos L, Messias Fde M, Lopes-Martins RA (2012) Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med Sci. 2012;15:71–78. https://doi.org/10.1007/s10103-011-0906-1
Alves AC, Vieira RP, Leal-Junior EC, Dos Santos SA, Ligeiro AP, Albertini R, Junior JA, de Carvalho PD (2013) Effect of low level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther 15(5):R116
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for conducting this study.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This experimental work was approved by Cairo University IACUC with approval number of CU/IF/87/19/2020. All of the experimental procedures were carried out in accordance with international standards for the care and use of laboratory animals and performed in accordance with the advice provided in the most recent edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, USA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamed, E., Al Balah, O.F.A., Refaat, M. et al. Photodynamic therapy mediated by methylene blue-loaded PEG accelerates skin mouse wound healing: an immune response. Lasers Med Sci 39, 141 (2024). https://doi.org/10.1007/s10103-024-04084-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04084-1