Abstract
This review aims to assess the efficacy and safety of laser therapy in managing scars resulting from cleft lip and/or palate (CL/P) repair surgeries, as well as to determine the optimal timing for intervention. A systematic search was conducted across four databases using a predefined search strategy. Studies included were randomized controlled trials, non-randomized studies, and case series focusing on laser therapy for CL/P scars. Data extraction and analysis were performed using Revman Software. A total of two randomized controlled trials, four non-randomized studies, and three case series were included in the analysis. The fractional CO2 laser was the most commonly utilized type of laser. Following laser therapy, there was a significant decrease in Vancouver Scar Scale (VSS) scores by 4.05 (95% CI, 2.10–5.99). Meta-analysis revealed that laser treatment groups exhibited a significantly lower mean VSS score (1.3; 95% CI, 0.02–2.67) compared to control groups. Moreover, initiating laser therapy intervention at one month postoperatively resulted in a significantly lower VSS score compared to initiation at three months postoperatively (difference of 1.70; 95% CI, 1.33–2.08). No severe complications were reported. Laser therapy demonstrates effectiveness and safety in improving CL/P scars, with earlier intervention yielding greater benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cleft lip and/or palate (CL/P) represents one of the most prevalent congenital deformities globally, affecting approximately 1 in 600 live births [1]. Although surgical intervention is the cornerstone for this malformation, the resulting scars after surgery affect aesthetics and functional outcomes, which may further hamper individuals’ psychological well-being. Current treatments for improving surgical scars involve secondary surgery [2], silicone-based products [3], or botulinum toxin type A injection [4]. However, new methods and techniques with less invasiveness or better efficacy to eliminate scars are still needed.
In recent years, the clinical application of laser therapy has expanded rapidly, offering a non-invasive or minimally invasive option for treating various dermatological issues [5, 6]. Laser therapy has emerged as a promising adjunctive technique for the prevention and treatment of surgical scars [7]. Previous studies have demonstrated that laser therapy can improve tissue microcirculation through vasodilatation, angiogenesis, and rebuilding collagen fibers, thereby modulating the wound healing process and potentially improving scar quality [8, 9].
Although laser therapy has gradually gained attention in scar treatment, its specific application and safety in cleft lip and palate (CL/P) remain relatively underexplored. While preliminary studies indicate its potential advantages, a comprehensive assessment of existing literature is necessary to determine the true efficacy of laser therapy in this population. Therefore, this systematic review and meta-analysis aim to comprehensively explore the existing evidence regarding the use of laser therapy for preventing and treating CL/P scar formation.
Method
Following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol, this systematic review was duly registered with the PROSPERO database (registration number: CRD42024475312).
Search strategy
A comprehensive search of the literature was conducted on February 22, 2024, across PubMed, Web of Science, Embase, and the Cochrane Library. The search terms included cleft lip and/or palate, scar, and laser, along with their respective synonyms.
Eligibility criteria
Inclusion criteria included full-text, peer-reviewed, and original studies, which investigate the efficacy of laser therapy in treating postoperative scars associated with CL/P. There were no restrictions based on publication time, language, or laser type. Reviews, conference abstracts, animal experiments and duplicated data were excluded.
Study selection
Two researchers (Y.S and Z.L) independently performed the initial screening of titles and abstracts from the identified literature. Subsequent full-text assessments were conducted on potentially relevant studies to ascertain their eligibility, and any discrepancies were resolved through mediation by a third reviewer (X.L).
Data extraction
Independent data extraction was performed by two investigators (Y.S and Z.L) from the included studies, involving the collection of article publication details, baseline characteristics, intervention specifics, outcomes, and follow-up information. The integrated data were integrated using Microsoft Word and subsequently cross-referenced between the two reviewers. Any disparities were resolved through discussion, with potential adjudication by a third reviewer (W.T).
Data analysis
A descriptive analysis of the studies was initially conducted. The inverse variance method was utilized to calculate the overall effect estimate. A random-effects model was applied if significant heterogeneity was detected, otherwise a fixed-effects model was utilized. Heterogeneity was evaluated using Cochran's Q and the I2 statistic, with I2 values exceeding 50% or P-values below 0.1 indicating significant heterogeneity. The differences in Vancouver Scar Scale (VSS) scores before and after the treatment were the main indicator for the treatment efficacy, the same as the differences in VSS scores between laser treatment and control group. Comparison of laser therapy performed in different postoperative time was also conducted to investigating the optimal timing of treatment. The continuous data was used with mean and standard deviation (SD), expressing with mean differences (MD) and calculated the 95% confidence intervals (CI). Publication bias was assessed using funnel plots. Comparative studies were separately analyzed. The meta-analyses were performed using Revman Software (version 5.4).
Scar pathology
The normal progression of wound healing consists of three recognized phases: the initial inflammatory phase, occurring within 1–3 days post-injury, characterized by activation of the extrinsic clotting cascade and formation of a fibrin plug; the subsequent proliferative phase, spanning from 4 to 21 days post-injury, marked by granulation tissue formation, synthesis of collagen III and extracellular matrix, and angiogenesis; and the final remodeling phase, extending from 21 days to 1 year post-injury, involving granulation tissue remodeling, collagen I production, and immature blood vessels regression [10].
Risk of bias assessment
Bias risk assessment for comparative studies adhered to the guidelines outlined in the Cochrane Handbook for Systematic Reviews (http://www.cochranelibrary.com/). Single-arm studies were categorized as high-risk for bias. Two reviewers (Y.S and Z.L) independently performed the assessment, with any disagreements resolved through mediation by a third reviewer (W.T).
Result
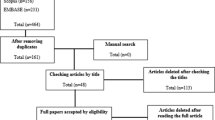
A total of 135 articles were retrieved from database searching. With duplicate removal, 93 articles were preliminary screened with title and abstract. Among them, eleven potentially eligible articles were identified for full-text retrieval, of which nine articles were ultimately included in the analysis. The detailed screening flow chart was presented in Fig. 1.
Among the 9 articles, there were 2 randomized controlled trials (RCTs) [8, 11], 4 non-randomized comparative studies [9, 12,13,14], and 3 prospective cohort [15,16,17] studies. Four studies were from China [8, 11, 13, 14], 3 studies from Egypt [9, 12, 17], 1 from Iran [16] and 1 from Italy [15]. This study reviewed a total of 451 patients, ranging in age from 3 months to 47 years, with a slight male predominance (58.8%). Based on available data, there were at least 25 cases of bilateral CL/P and 133 cases of unilateral CL/P. Of these, 204 patients underwent primary repair surgery, while 105 patients received secondary repair surgery. Three studies limited patients’ Fitzpatrick skin phototype to III or IV type. Detailed information for each eligible study is presented in the Table 1.
Two comparative studies [8, 11] exhibited a high risk of performance bias but were rated as low risk in other domains. Meanwhile, two other comparative studies [9, 13] were classified as high risk primarily due to their inadequate control over selection bias and performance bias. The remaining two comparative studies [12, 14] did not display the randomization and blinding process, thereby the relative bias risk is unclear (Fig. 2).
Laser type
The fractional CO2 laser was the most commonly used laser type (n = 5), typically administered once every 4 weeks, with a total treatment course of 5–7 sessions. In a self-controlled study [11] the 595 nm pulsed dye laser (PDL) was applied every two weeks for 5 sessions. Low power diode laser 806 nm was utilized in a study with a denser interval (three times per week) and more sessions (12). Nocini et al. [15] used Er: YAG laser once every 3 months for two sessions. Peng et al. [14] combined intense pulsed laser (IPL) and fractional CO2 laser, administering the former once a month and the latter once every three months, resulting in a total treatment duration of six months. The intervention detail of each study was presented in the Tables 2 and 3.
VSS
Six articles recorded the VSS scores change after laser treatment, with only four of them [8, 12, 14, 17] reporting mean values and standard deviations, which were suitable for data integration. All studies observed a significant decrease in scores after laser therapy, with an overall mean difference of 4.05 (95% CI, 2.10–5.99) (Fig. 3). The overall heterogeneity was significant with a I2 of 99%. The funnel plot displayed an asymmetric distribution, suggesting some publication bias (Supplemental Fig. 1). Further subgroup analysis according to the laser types showed that for factional CO2 laser treatment, the mean difference is 3.36 (95% CI, 2.45–4.07). One study [14] combined IPL and fractional CO2 laser and reported a difference of 7.60 (95% CI, 7.24–7.96).
We also conducted separate meta-analysis for comparative results. One study [8] set three experimental groups with different intervention initiation time, while another study [12] set two experimental groups. Compared to control group (2 studies [12, 14] used scar creams and silica gel, 1 studies [8] did not provide detailed descriptions), the laser treatment groups have a lower mean VSS score of 1.34 (95% CI, 0.02–2.67) (Fig. 4).
Two studies did not report data in the form of mean and SD. One study [11] conducted a randomized, self-controlled trial aimed at evaluating the efficacy of 595-nm PDL. At the 6-month follow-up, the relative change in VSS was 0.116 ± 0.336, significantly lower than that in the control group. An open-label study [9] compared low-power 806-nm diode laser with the control group. The median VSS score of laser group (median = 3) was significantly lower than the control group (median = 6.5).
Scar width/area/thickness
Two studies assessed the scar width. Shadad et al. [12] found that the scar width in the early intervention group (2.51 ± 0.64) was significantly lower than that in the late intervention group (3.17 ± 0.54) and the control group (3.27 ± 0.48). However, in Mohsen's study, there was no significant difference in scar width between the low-power diode laser treatment group and the control group [9]. Mohsen et al. [9] also utilized ultrasound to measure the scar thickness and observed a reduction in scar thickness on the 14th day of laser treatment [9]. Detailed data on scar width or thickness were not provided in this study.
In Chi's self-controlled study, a 3dMD photographic measurement system was used to calculate scar area [11]. The mean area on the laser side (24.20 ± 10.95 mm2) was significantly smaller than that on the control side (31.19 ± 14.51 mm2) after treatment.
Other indicators
Li et al. [13] utilized a subjective rating scale, grading the efficacy to obviously effective, effective and ineffective according to the change of pliability, color and thickness. In the laser treatment group, there was a notable prevalence of patients exhibiting obviously effective or effective efficacy compared to those in the control group. Quartile grading scale was employed in Jahanbin’s study [16] to assess the improvement of scar texture. 0 means minimal to no improvement while 3 represents near total improvement. The results were evaluated by two blind dermatologists and showed a mean of 1.29 ± 0.86, a median of 1.25 ± 1.38 after treatment. Nocini et al. [15] used patient satisfaction questionnaire as the primary indicator and the average satisfaction level is 8.8 (1: bad;10: high).
Some adjuvant assessments also performed in some studies as efficacy indicators, such as visual analog scale (VAS) [12], patient scar assessment questionnaire (PSAQ) [11, 16]. All the relative results support the efficacy of laser therapy in improving CL/P scar.
Optimal begin time of laser therapy
Three cohorts [9, 11, 12] started intervention at the proliferative phase and five cohorts [8, 12, 13] initiated treatment at the remodeling phase. All cohorts that commenced intervention during the proliferative phase exhibited a significant therapeutic effect. Two cohorts [8] that initiated treatment during the proliferative phase (one at 3 months and the other at 6 months after surgery) demonstrated similar improvement post-treatment compared to the control group. The resting cohorts showed significant improvement. A meta-analysis was performed to assess the efficacy of intervention initiation approximately one month postoperatively compared to initiation at three months postoperatively. The results revealed that the VSS score was significantly lower in the former group, with a difference of 1.70 (95% CI, 1.33–2.08) (Fig. 5).
Complications
No severe complications were reported in included studies. However, transient erythema or swelling following Er:YAG laser treatment is commonly observed [15], with sporadic reports in other types of laser therapy. Additionally, Mossaad et al. documented patients complaining of discomforting pain during the treatment [17]. Overall, laser therapy is considered a safe treatment for preventing and treating CL/P scars.
Discussion
This study represents the first systematic review and meta-analysis of laser therapy for scar management following CL/P repair surgery. Through comprehensive analysis of nine original articles, our findings indicate that various types of laser treatments are effective in improving scars post-CL/P repair surgery, exhibiting excellent safety, with early intervention showing superior efficacy.
The objective of treating cleft lip scars is to prevent scar hypertrophy or contracture and correct secondary deformities [3]. Specific repair methods vary depending on the severity and extent of the defect. These methods range from surface adjustments to surgical interventions, which involve deeper layers, such as the orbicularis oris muscle. Laser therapy primarily targets surface repair, facilitating wound healing and preventing abnormal scar formation. The initial descriptions of laser scar treatment can be traced back to 1993 when Alster et al. first introduced the use of pulsed dye laser for treating erythematous and hypertrophic scars [18]. Then Er:YAG resurfacing lasers and full surface ablative CO2 lasers were developed, and the former was the first laser type applied in improving CL/P scar [15]. Innovations in fractional laser technology thereafter enhanced the safety of laser treatments and facilitated their application in scar management [19].
Different lasers target various biological components or process. PDL is a vascular laser, with its wavelength selectively absorbed by oxygenated hemoglobin [7]. The laser produces vascular injury and coagulates the microvasculature of the scar, thereby deprive nutrients and potentially impairing fibroblast proliferation. Chi et al. [11] treated CL/P scars with PDL two weeks postoperatively, when angiogenesis is extremely active, demonstrating significant improvement. Er: YAG (2,940 nm) and CO2 (10,600 nm) lasers are ablative resurfacing lasers, with their wavelengths selectively absorbed by water. These lasers heat the affected epidermal and dermal regions above 100 °C, vaporizing the target tissue. Er:YAG laser operates at a wavelength of 2,940 nm, showing a high absorption coefficient for water. This enables the ablation of thin tissue layers (5–20 μm) and minimizes residual heat damage, potentially resulting in pinpoint bleeding [7]. On the contrary, the CO2 laser exhibits weaker water absorption, causing the vaporization of thicker tissue layers (20–30 μm) with a residual thermal injury zone of 50–130 μm, generating a bloodless operative field. Thus, Er:YAG laser treatment usually accompanied with erythema or swelling but these symptoms are only sporadic in studies using CO2 fractional resurfacing technology. Fractional CO2 laser is the most common laser used in CL/P scar treatment based on our review. The laser can inhibit angiogenesis in the early stage and collagen production in the remodeling stage, thereby improve the scar formation [20]. Our results show the early intervention (within 1 month after surgery) is better than late intervention, in line with the 2020 consensus. Peng et al. [14] combined the intense pulsed light and CO2 laser; the former degrading the microvasculature and the latter stimulating collagen remodeling. As Peng's theory, IPL is administered monthly, starting two weeks postoperatively, to diminish scar vascularization. CO2 laser therapy initiates one month postoperatively and recurs every three months to enhance treatment efficacy. The experimental group showed a significantly low VSS score (0.16 ± 0.55) at the 6-month follow-up, indicating excellent outcomes with early combined intervention. Although different lasers are effective in CL/P treatment, the optimal treatment pattern still need further exploration: Is combination of different lasers better than just one laser? What is the best laser type for early intervention of CL/P scar?
Currently, the assessment of scar severity primarily relies on evaluating texture, color, size, and other attributes [4]. Assessment tools include both subjective and objective evaluation methods. The most commonly used subjective assessment tool is the VSS, which includes four subdomains: pigmentation, vascularity, pliability, and height [21]. It employs a semi-quantitative method to assess scars, with scores ranging from 0 to 13, where 0 represents normal and 13 represents severe scar. PSAQ was developed in 2009 [22], comprising four dimensions: appearance, consciousness, satisfaction with appearance, and satisfaction with symptoms. This scale has been subsequently modified and applied in later studies [11, 16]. Objective assessment of scars involves techniques such as ultrasonographic measurement of scar thickness [9] and photographic measurement of scar area [11]. However, there is currently no widely accepted comprehensive objective scar assessment tool.
This systematic review and meta-analysis have several limitations. Firstly, the outcome indicators and data presentation varied in different studies which posed challenges in uniformly interpreting the results. Secondly, most of included studies only had follow-up periods ranging from 6 to 12 months, necessitating longer follow-up durations to explore long-term effects and detect recurrence. Thirdly, the number of high-quality randomized controlled trials included was quite limited. We anticipate more high-quality studies in the future to compare the therapeutic effects of different laser therapies and assess the advantages and disadvantages of various laser types.
Conclusion
This meta-analysis supports the use of laser therapy for aesthetically improving scars following CL/P repair surgery, while also proving its sufficient safety. The effects of laser therapy on CL/P scars, as evaluated by other indicators, such as parameters of scar size and some other subjective rating scales, have also been comprehensively described. Notably, early intervention within one month postoperatively is more beneficial. Further high-quality RCTs are expected to explore the optimal laser therapy pattern in the future.
Data availability
The data in this manuscript are extracted from the published primary studies, all of which are duly referenced within the text and fully listed in the reference section. The data extracted are summarized in the tables, figures, and supplementary material within the manuscript. Original detailed excel sheets can be obtained from the corresponding authors if requested.
References
Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC (2009) Cleft lip and palate. Lancet 374(9703):1773–85. https://doi.org/10.1016/s0140-6736(09)60695-4
Monson LA, Khechoyan DY, Buchanan EP, Hollier LH Jr (2014) Secondary lip and palate surgery. Clin Plast Surg 41(2):301–309. https://doi.org/10.1016/j.cps.2013.12.008
Bartkowska P, Komisarek O (2020) Scar management in patients after cleft lip repair-Systematic review Cleft lip scar management. J Cosmet Dermatol 19(8):1866–1876. https://doi.org/10.1111/jocd.13511
Ji Q, Tang J, Hu H, Chen J, Cen Y (2022) Botulinum toxin type A for preventing and treating cleft lip scarring-A systematic review and meta-analysis. J Cosmet Dermatol 21(6):2331–2337. https://doi.org/10.1111/jocd.14941
Marson JW, Baldwin HE (2019) An overview of acne therapy, part 1: topical therapy, oral antibiotics, laser and light therapy, and dietary interventions. Dermatol Clin 37(2):183–193. https://doi.org/10.1016/j.det.2018.12.001
Paasch U, Zidane M, Baron JM et al (2022) S2k guideline: Laser therapy of the skin. J Dtsch Dermatol Ges 20(9):1248–1267. https://doi.org/10.1111/ddg.14879
Kauvar ANB, Kubicki SL, Suggs AK, Friedman PM (2020) Laser therapy of traumatic and surgical scars and an algorithm for their treatment. Lasers Surg Med 52(2):125–136. https://doi.org/10.1002/lsm.23171
Chi H, Zhao X, Shen L, Liu Y, Cai M (2023) Optimal timing of fractional CO2 laser on cleft lip scars: a single-blind randomized controlled cohort study. Dermatol Surg 49(2):145–148. https://doi.org/10.1097/DSS.0000000000003688
Mohsen MA, Saafan AMEH, El-Basiouny MS et al (2023) Evaluating the effect of low power diode laser 806 nm on the healing of unilateral cleft lip scar: an open-label comparative study. Cleft Palate Craniofac J 60(1):21–26. https://doi.org/10.1177/10556656211053536
Profyris C, Tziotzios C, Do Vale I (2012) Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol 66(1):1–10; quiz 11–2. https://doi.org/10.1016/j.jaad.2011.05.055
Chi H, Peng H, Zhao X, Zhou G, Shen L, Cai M (2024) The effectiveness of 595-nm pulsed dye laser for the treatment of bilateral cleft lip scars in Asian patients: a 6-month prospective, randomized, self-controlled trial. Adv Wound Care. https://doi.org/10.1089/wound.2023.0106
Shadad M, Ali WM, Fayyaz GQ, El-Shazly M (2021) Use of fractional CO2 laser in cleft lip scars: does it make a difference? Article Annals of plastic surgery 86(5):536–539. https://doi.org/10.1097/SAP.0000000000002511
Li YY, Wu M, Liu MX et al (2020) Effect of CO2 fractional laser on the early control of scar post-secondary repair in patients with a cleft lip. Huaxi Kouqiang Yixue Zazhi 38(6):657–661. https://doi.org/10.7518/hxkq.2020.06.009[InChinese]
Peng L, Tang S, Li Q (2018) Intense pulsed light and laser treatment regimen improves scar evolution after cleft lip repair surgery. J Cosmet Dermatol 17(5):752–755. https://doi.org/10.1111/jocd.12684
Nocini PF, D’Agostino A, Trevisiol L, Bertossi D (2003) Treatment of scars with er:YAG laser in patients with cleft lip: a preliminary report. Cleft Palate Craniofac J 40(5):518–522. https://doi.org/10.1597/1545-1569(2003)040
Jahanbin A, Eslami N, Layegh P et al (2019) Fractional CO2 laser treatment for post-surgical lip scars in cleft lip and palate patients. Lasers Med Sci 34(8):1699–1703. https://doi.org/10.1007/s10103-019-02819-z
Mossaad A, Kotb A, Abdelrahaman M, Ahmady HA (2018) Post-surgical repair of cleft scar using fractional CO2 laser. Open Access Maced J Med Sci 6(7):1231–1234. https://doi.org/10.3889/oamjms.2018.250
Alster TS, Kurban AK, Grove GL, Grove MJ, Tan OT (1993) Alteration of argon laser-induced scars by the pulsed dye laser. Lasers Surg Med 13(3):368–373. https://doi.org/10.1002/lsm.1900130314
Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR (2004) Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 34(5):426–438. https://doi.org/10.1002/lsm.20048
DeBruler DM, Blackstone BN, Baumann ME et al (2017) Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med 49(7):675–685. https://doi.org/10.1002/lsm.22666
Baryza MJ, Baryza GA (1995) The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil Sep-Oct 16(5):535–538. https://doi.org/10.1097/00004630-199509000-00013
Durani P, McGrouther DA, Ferguson MW (2009) The Patient Scar Assessment Questionnaire: a reliable and valid patient-reported outcomes measure for linear scars. Plast Reconstr Surg 123(5):1481–1489. https://doi.org/10.1097/PRS.0b013e3181a205de
Funding
The work was supported by National High Level Hospital Clinical Research Funding (fund no. 2022-PUMCH-A-208).
Author information
Authors and Affiliations
Contributions
Conception and design: Yixin Sun, Ziming Li, Wenyun Ting, Xiao Long, Nanze Yu, Jiuzuo Huang.
Data collection and analysis: Yixin Sun, Ziming Li, Xiaoyu Qi, Binghan Wang.
Writing – original draft: Yixin Sun, Ziming Li, Xiaoyu Qi, Binghan Wang.
Writing – review & editing: Wenyun Ting, Xiao Long, Nanze Yu, Jiuzuo Huang.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors have no financial or other conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Y., Li, Z., Qi, X. et al. Laser therapy for treating cleft lip or/and palate scarring—a systematic review and meta-analysis. Lasers Med Sci 39, 160 (2024). https://doi.org/10.1007/s10103-024-04082-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04082-3