Abstract
Low-level laser therapy (LLLT) has been a treatment modality by many androgenetic alopecia (AGA) patients in recent years. It remained unclear as to how long the treatment regime should be maintained, and which characteristics of patients should this be recommended. A real-world study was carried out with an FDA-cleared low-level laser helmet for 1383 patients. Ordinal logistic regression analysis with propensity score matching (PSM) was used to investigate the factors related to efficacy assessment. More than 80% of users were between 18 and 40 years old. The median use times were 133 for mild AGA patients and 142 for moderate-to-severe AGA patients, which equated to 38 weeks and 40 weeks, respectively. The overall clinical effectiveness was nearly 80%. PSM analysis revealed that gender (P = 0.002), use period (P = 0.068), scalp conditions with dandruff, rash, and itchy symptoms were associated with the grading of efficacy assessment. Male users (ordinal OR: 1.35, CI: (1.01, 1.79)); use for more than 180 times or use period for 1 year (ordinal OR: 1.40, CI: (1.11, 1.96)); and those with scalp dandruff (ordinal OR: 1.34, CI: (1.01, 1.87)), rash (ordinal OR: 1.47, CI: (1.04, 2.07)), and itchy symptoms (ordinal OR: 1.51, CI: (1.12, 2.03)) had better efficacy assessments. The recommended treatment regime with low-level laser helmet was more than 1 year or 180 use times. Male patients with dandruff, rash, and itchy symptoms in scalps tended to have a better efficacy assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Androgenetic alopecia (AGA) is the most common type of nonscarring progressive hair loss, characterized by gradual follicular miniaturization presenting a receding frontal hairline or notable hair loss around the vertex in men, and a diffuse thinning of the hair on the crown in women [1, 2]. Although the prevalence of AGA in Chinese men and women is comparatively lower than that in Caucasians, there is an evident tendency that prevalence of AGA has been gradually increasing with early onset in recent years [3, 4]. AGA has psychosocial distressing impact on alopecia patients that diminish their self-confidence especially among those young with more severe balding [5, 6]. Currently, the common treatment options for AGA include hair transplantation, oral finasteride, topical minoxidil. In addition, there are some additional treatments, such as injection of platelet-rich plasma (PRP) and low-level laser therapy (LLLT) [7,8,9].
In recent years, LLLT has been a popular treatment option by many AGA patients in China [10]. At present, there are several commercially available devices designed for home use in China, among which, the iHelmet (Slinph Technologies Co., Ltd, Shenzhen, China), an FDA-cleared (K162782) helmet-like device with lasers and light-emitting diodes, has been promoted to several thousands of users in the past 5 years.
Several previous studies suggested that low-level laser treatment would be an effective option to treat pattern hair loss in both men and women [11,12,13,14]. There were some limitations in these studies: (1) small sampling (2–225 subjects); (2) short-term study with maximum period of 26 weeks; (3) selection bias. Real-world studies use information from real clinical setting and seek to provide evidence complementary to that provided by randomized controlled trials (RCTs). To fill in the above research gap, this study aimed to determine the efficacy assessment of LLLT for 1383 Chinese AGA patients (median treated period with 38 weeks) with a real-world study using propensity score matching (PSM)analysis.
Methods
LLLT device and data acquisition
The iHelmet is an LLLT device with light sources consisting of two hundred laser diodes (5 mW, 650 nm) in seven scalp sections. The lasers for each device were identical in power output, and the treatment frequency was 20 min every other day. All the information including baseline data and treatment data were acquired by an APP named “iHelmet,” which could be downloaded through “Google Play” or “App Store”.
The independent variables included age groups, gender, marital status, family history with AGA, use duration, combined with medical treatment record, and scalp conditions. The severity of AGA was graded by participants first, and double-checked by two dermatologists later with the upgraded pictures according to basic and specific (BASP) classifications. The severity was dichotomized as mild (L, M0, C0, M1, C1, F1, V1) and moderate-to-severe groups (M2, C2, F2, V2, and M3, C3, F3, V3, U1∼3) [15]. The protocol was approved by the Ethics Committee of Southern Medical University, China.
Treatment regime and efficacy assessment
There were three treatment options in this research including LLLT monotherapy, LLLT concomitant with topical minoxidil, and LLLT concomitant with oral finasteride.
The efficacy was assessed with six items: (1) whether the scalp oil secretion reduced? (2) whether the scalp dandruff reduced? (3) whether the scalp rash reduced? (4) whether the daily hair loss reduced? (5) whether new hair regrown or hair density increased? (6) whether hair changed into thicker? For the above six questions, 1-point score was given if the answer was “yes,” and a zero-score given for those questions if the answer was “no.” The overall efficacy assessment was graded by summarizing the total score for six items, “not effective” for that total score was zero, “moderately effective” for those scored 1 to 3, and “significantly effective” for those scored 4 to 6. The efficacy was assessed by participants first, double-checked by doctors with trichoscopy.
Statistical analysis
R software (R language version 3.5.2) and EpiDisplay Package (version 3.5.0.1) were used in data analysis. Frequencies and percentages were used for descriptive variables. The differences between qualitative variables were assessed with the chi-square test or Fisher’s exact test. To reduce the selection bias conferred by potential confounding factors, a propensity score matching (PSM) analysis was performed with age, gender, and marital status matched between the mild and moderate-to-severe groups. The logit of the propensity score was nearest-neighbor matched in a 1:1 manner. Ordinal logistic regression analyses were used to model the association between the grading of efficacy assessment, demographic factors, and treatment regime. All values were two-tailed, and a p-value < 0.05 was considered to indicate statistical significance.
Results
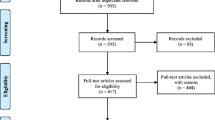
In total, 6833 AGA patients used LLLT with iHelmet; 1746 of them submitted complete information during the treatment. Three hundred sixty-three were excluded for age < 18 or age > 60; 1383 of them were included in the final analysis. To reduce the selection bias conferred by age, gender, and marital status, a PSM was performed with age, gender, and marital status matched between the mild (N = 455) and moderate-to-severe groups (N = 455), as shown in Fig. 1.
As shown in Table 1, the distribution of AGA patients using LLLT helmet in mild and moderate-to-severe groups was similar (P = 0.335); the majority of users (more than 80%) were between 18 and 40 years old. However, the gender difference existed (P = 0.007), more than two-thirds of users were male, and females in moderate-to-severe groups were more likely to use LLLT helmet whereas less likely in the mild groups. The median use times were 133 for mild AGA patients and 142 for moderate-to-severe AGA patients, which equated to 38 weeks and 40 weeks, respectively. Almost 60% of them had a family history of AGA, and more than half of them were single. Over 80% of the users presented with oil scalp. Generally, the overall efficacy assessment concluded that treatment with LLLT helmet was moderately effective for 51.9% of users with mild AGA and 57.4% with moderate-to-severe AGA, significantly effective for 27.7% users with mild AGA and 20.0% with moderate-to-severe AGA. The overall clinical effectiveness was nearly 80% by summing up the rate for moderately effective and significantly effective.
After PSM by age, gender, and marital status, a PSM cohort consisted of 455 users with mild AGA and 455 with moderate-to-severe AGA are analyzed in Table 2. As shown in this table, the factors including age groups (P = 0.948), family history with AGA (P = 0.457), marital status (P = 0.747), treatment regime (P = 0.402), and oil scalp (P = 0.438) were not associated with the grading of efficacy assessments, while the other factors such as gender (P = 0.002), use period (P = 0.068), scalp conditions with dandruff, rash, and itchy symptoms were associated with the grading of efficacy assessment.
To further analyze the factors associated with the efficacy assessments, an ordinal logistic regression analysis is summarized in Table 3. Set the grading “not effective” as reference; the factors of male (P = 0.02), use period of more than 180 times (P = 0.03), scalp condition with dandruff (P = 0.04), rash (P = 0.01), and itchy symptoms (P = 0.003) were positively associated with efficacy assessments. It was likely that the male users (ordinal OR: 1.35, CI: (1.01, 1.79)); use for more than 180 times or use period for more than 1 year (ordinal OR: 1.40, CI: (1.11, 1.96)); and those with scalp dandruff (ordinal OR: 1.34, CI: (1.01, 1.87)), rash (ordinal OR: 1.47, CI: (1.04, 2.07)), and itchy symptoms (ordinal OR: 1.51, CI: (1.12, 2.03)) had better efficacy assessments.
Discussion
Although Endre Mester firstly documented that accelerated hair regrowth in shaved mice after exposure to a low-power 694-nm ruby laser in 1967 [16], which discovered the potential of low-level laser therapy for hair loss, it was not until in the year of 2009 that the first RCT on the role of LLLT in AGA was carried out for Hair Max Laser Comb to receive FDA clearance [17]. In the past decade, several other studies confirmed the effectiveness of LLLT on increasing hair count and hair thickness. However, these studies had some limitations. Firstly, the study enrolled few patients, and the treatment periods with LLLT were comparatively short. Secondly, these researches had not taken the scalp conditions into study. Above all, most of these studies had strict inclusion and exclusion criteria, which meant that trial populations were not good representative of the real alopecia population in real-world practice. By far, there was not any real-world study with larger sample size.
As a typical real-world study, this study filled in the above research gaps. The iHelmet made by Slinph Technology Co., Ltd, Shenzhen, China, was an FDA-cleared (K162782) helmet-like LLLT device. All data for several thousand users were extracted from the APPs linked to iHelmet the in the past 5 years. The median treatment periods were equal to 38 weeks and 40 weeks for mild and moderate-to-severe AGA patients. The study adopted a comprehensive efficacy assessment including six items. The overall efficacy assessment concluded that treatment with LLLT helmet were moderately effective for 51.9% of users with mild AGA and 57.4% with moderate-to-severe AGA, significantly effective for 27.7% and 20.0% of them, respectively; the results were better than the self-assessment of efficacy in the research conducted by Jimenez et al. in 2014 [11]. In previous studies, LLLT with a topical combination of 0.25% finasteride and 3% minoxidil improved efficacy in the treatment of FPHL with an additional benefit of increasing hair diameter [18]. Combined treatment was better in reducing oil secretion, improving hair diameter and hair density [19]. No synergistic effects were found in efficacy assessments among LLLT monotherapy, LLLT concomitant with minoxidil, and LLLT concomitant with finasteride in this study, which were different from previous research results [20].
Meanwhile, this study answered the questions on what characteristics of AGA patients should be recommended to LLLT, how long would it take for LLLT to get prime treatment effects. This study proved that male users; use for more than 180 times or use period for 1 year; and those with dandruff, rash, and itchy symptoms in scalps were more likely to have better efficacy assessments. The findings had important significance in clinical setting.
In conclusion, this was a real-world study on LLLT in the treatment of AGA with a big sample size. The overall clinical effectiveness with iHelmet for AGA was nearly to 80%. The recommended treatment regime with low-level laser helmet was more than 1 year or 180 use times. Male patients with dandruff, rash, and itchy symptoms in scalps tended to have a better efficacy assessment.
Change history
23 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10103-022-03526-y
References
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, Sansone A, Lombardo F (2017) Androgenetic alopecia: a review. Endocrine 57(1):9–17. https://doi.org/10.1007/s12020-017-1280-y
Bhat YJ, Saqib NU, Latif I, Hassan I (2020) Female pattern hair loss-an update. Indian Dermatol Online J 11(4):493–501. https://doi.org/10.4103/idoj.IDOJ_334_19
Ding Q, Xu YX, Sun WL, Liu JJ, Fan WX (2020) Early-onset androgenetic alopecia in China: a descriptive study of a large outpatient cohort. J Int Med Res 48(3):030006051989719
Jang WS, Son IP, Yeo IK, Park KY, Li K, Kim BJ, Seo SJ, Kim MN, Hong CK (2013) The annual changes of clinical manifestation of androgenetic alopecia clinic in korean males and females: a outpatient-based study. Ann Dermatol 25(2):181–188. https://doi.org/10.5021/ad.2013.25.2.181
Ghimire RB (2018) Impact on quality of life in patients who came with androgenetic alopecia for hair transplantation surgery in a Clinic. JNMA J Nepal Med Assoc 56(212):763–765
Gupta S, Goyal I, Mahendra A (2019) Quality of life assessment in patients with androgenetic alopecia. Int J Trichology 11(4):147–152. https://doi.org/10.4103/ijt.ijt_6_19
Qu Q, Shi P, Yi Y, Fan Z, Liu X, Zhu D, Chen J, Ye K, Miao Y, Hu Z (2019) Efficacy of platelet-rich plasma for treating androgenic alopecia of varying grades. Clin Drug Investig 39, 865–872 (2019). https://doi.org/10.1007/s40261-019-00806-4
Afifi L, Maranda EL, Zarei M, Delcanto GM, Falto-Aizpurua L, Kluijfhout WP, Jimenez JJ (2017) Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg Med 49(1):27–39. https://doi.org/10.1002/lsm.22512
Adil A, Godwin M (2017) The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol 77(1):136-141.e135. https://doi.org/10.1016/j.jaad.2017.02.054
Zhou Y, Chen C, Qu Q, Zhang C, Wang J, Fan Z, Miao Y, Hu Z (2020) The effectiveness of combination therapies for androgenetic alopecia: a systematic review and meta-analysis. Dermatol Ther:e13741. https://doi.org/10.1111/dth.13741
Jimenez JJ, Wikramanayake TC, Bergfeld W, Hordinsky M, Hickman JG, Hamblin MR, Schachner LA (2014) Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol 15(2):115–127. https://doi.org/10.1007/s40257-013-0060-6
Avram MR, Rogers NE (2009) The use of low-level light for hair growth: part I. J Cosmet Laser Ther 11(2):110–117. https://doi.org/10.1080/14764170902842531
Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ (2018) Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci 33(2):425–434. https://doi.org/10.1007/s10103-017-2385-5
Suchonwanit P, Chalermroj N, Khunkhet S (2019) Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci 34(6):1107–1114. https://doi.org/10.1007/s10103-018-02699-9
Lee WS, Lee HJ, Choi GS, Cheong WK, Chow SK, Gabriel MT, Hau KL, Kang H, Mallari MR, Tsai RY, Zhang J, Zheng M (2013) Guidelines for management of androgenetic alopecia based on BASP classification–the Asian Consensus Committee guideline. J Eur Acad Dermatol Venereol 27(8):1026–1034. https://doi.org/10.1111/jdv.12034
Mester E, Szende B, Gärtner P (1968) The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 9(5):621–626
Leavitt M, Charles G, Heyman E, Michaels D (2009) HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig 29(5):283–292. https://doi.org/10.2165/00044011-200929050-00001
Suchonwanit P, Iamsumang W, Rojhirunsakool S (2019) Efficacy of topical combination of 0.25% finasteride and 3% minoxidil versus 3% minoxidil solution in female pattern hair loss: a randomized, double-blind, controlled study. Am J Clin Dermatol 20(1):147–153. https://doi.org/10.1007/s40257-018-0387-0
Liu Y, Jiang LL, Liu F, Qu Q, Fan ZX, Guo Z, Miao Y, Hu ZQ (2021) Comparison of low-level light therapy and combination therapy of 5% minoxidil in the treatment of female pattern hair loss. Lasers Med Sci 36(5):1085–1093. https://doi.org/10.1007/s10103-020-03157-1
Munck A, Gavazzoni MF, Trüeb RM (2014) Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology 6(2):45–49. https://doi.org/10.4103/0974-7753.138584
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This study was reviewed and approved by the research ethics board at Nanfang Hospital affiliated to Southern Medical University, Guangzhou, China. All contents adhered to the tenets of the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Qiu, Yanhua Yi and Linlang Jiang are contributed equally to this work.
The original version of this article was revised: The phrase “The iHelmet is an LLLT device with a light source consisting of two hundred 5 mW laser diodes (650 nm) in seven scalp sections” under the Methods section should be “The iHelmet is an LLLT device with light sources consisting of two hundred laser diodes (5 mW, 650 nm) in seven scalp sections”. Also, under Background section in the 2nd paragraph “Slinph Technology Co., Ltd,” should be “Slinph Technologies Co., Ltd”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, J., Yi, Y., Jiang, L. et al. Efficacy assessment for low-level laser therapy in the treatment of androgenetic alopecia: a real-world study on 1383 patients. Lasers Med Sci 37, 2589–2594 (2022). https://doi.org/10.1007/s10103-022-03520-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-022-03520-4