Abstract
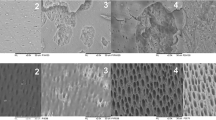
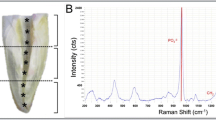
The purposes of this study were (1) to investigate the direct effect of an Er,Cr:YSGG laser on human apical papilla cell (APC) proliferation and mineralization and (2) to examine the effect of Er,Cr:YSGG laser, when applied to an ex vivo immature tooth model, on APC attachment. An Er,Cr:YSGG laser at various power outputs (0.1, 0.5, and 1 W) was used at different positions (2, 5, or 8 mm from the cells) to irradiate cultured APCs. APC proliferation and mineralization were assessed at various intervals. For the cell attachment evaluation, ex vivo tooth models containing dentin samples were irrigated with either EDTA or normal saline solution (NSS) and supplemented with laser activation. Fibronectin-positive-staining cells were counted and analyzed. The number of APCs was significantly greater when power outputs of 0.1 W and 0.5 W were used than when 1 W was used (P < 0.05). The close contact of laser application, at 2 and 5 mm, exerted a negative effect on cell proliferation at 24 and 48 h. The application at 8 mm did not show the deterioration effect. APC mineralization was reduced after laser irradiation, regardless of the power and the tip positioning, at 21 days. APC attachment in all laser-activated groups was significantly greater than in the groups without laser. The use of Er,Cr:YSGG laser significantly promoted APC attachment on the root canal dentin.




Similar content being viewed by others
References
Nakashima M, Akamine A (2005) The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 31:711–718
Katebzadeh N, Dalton B, Trope M (1998) Strengthening immature teeth during and after apexification. J Endod 24:256–259
Bose R, Nummikoski P, Hargreaves KM (2009) A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35:1343–1349
Diogenes AR, Ruparel NB, Teixeira FB, Hargreaves KM (2014) Translational science in disinfection for regenerative endodontics. J Endod 40:S52–S57. https://doi.org/10.1016/j.joen.2014.01.015
Diogenes A, Henry M, Teixeira F, Hargreaves K (2013) An update on clinical regenerative endodontics. Endodontic Topics 28:2–23
Kahler B, Kahler SL, Lin LM (2018) Revascularization-associated intracanal calcification: a case report with an 8-year review. J Endod 44:1792–1795. https://doi.org/10.1016/j.joen.2018.08.009
AAE (2016) AAE clinical considerations for a regenerative procedure revised 6–8–16. Book title.,
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651. https://doi.org/10.1016/j.joen.2008.03.001
Ruparel NB, de Almeida JF, Henry MA, Diogenes A (2013) Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod 39:357–363. https://doi.org/10.1016/j.joen.2012.10.027
Prompreecha S, Sastraruji T, Louwakul P, Srisuwan T (2018) Dynamic irrigation promotes apical papilla cell attachment in an ex vivo immature root canal model. J Endod 44:744–750. https://doi.org/10.1016/j.joen.2018.01.012
Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, Schmalz G (2016) EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J 49:581–590. https://doi.org/10.1111/iej.12492
Bolortuya G, Ebihara A, Ichinose S, Watanabe S, Anjo T, Kokuzawa C, Saegusa H, Kawashima N, Suda H (2011) Initial fibroblast attachment to erbium:YAG laser-irradiated dentine. Int Endod J 44:1134–1144. https://doi.org/10.1111/j.1365-2591.2011.01934.x
Talebi-Ardakani MR, Torshabi M, Karami E, Vajar N, Rezaei Esfahrood Z, Meimandi M, Mojahedi SM (2017) Comparison of Er:YAG laser and hand instrumentation on the attachment of cultured human gingival fibroblasts to periodontally involved root surfaces. J Lasers Med Sci 8:S51-s55. https://doi.org/10.15171/jlms.2017.s10
Blanken J, De Moor RJ, Meire M, Verdaasdonk R (2009) Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 1: a visualization study. Lasers Surg Med 41:514–519. https://doi.org/10.1002/lsm.20798
Bolhari B, Ehsani S, Etemadi A, Shafaq M, Nosrat A (2014) Efficacy of Er, Cr:YSGG laser in removing smear layer and debris with two different output powers. Photomed Laser Surg 32:527–532. https://doi.org/10.1089/pho.2014.3766
De Moor RJ, Blanken J, Meire M, Verdaasdonk R (2009) Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 2: evaluation of the efficacy. Lasers Surg Med 41:520–523. https://doi.org/10.1002/lsm.20797
Deleu E, Meire MA, De Moor RJ (2015) Efficacy of laser-based irrigant activation methods in removing debris from simulated root canal irregularities. Lasers Med Sci 30:831–835. https://doi.org/10.1007/s10103-013-1442-y
Peeters HH, Suardita K (2011) Efficacy of smear layer removal at the root tip by using ethylenediaminetetraacetic acid and erbium, chromium: yttrium, scandium, gallium garnet laser. J Endod 37:1585–1589. https://doi.org/10.1016/j.joen.2011.08.022
Bergmans L, Moisiadis P, Teughels W, Van Meerbeek B, Quirynen M, Lambrechts P (2006) Bactericidal effect of Nd:YAG laser irradiation on some endodontic pathogens ex vivo. Int Endod J 39:547–557. https://doi.org/10.1111/j.1365-2591.2006.01115.x
Dewsnup N, Pileggi R, Haddix J, Nair U, Walker C, Varella CH (2010) Comparison of bacterial reduction in straight and curved canals using erbium, chromium:yttrium-scandium-gallium-garnet laser treatment versus a traditional irrigation technique with sodium hypochlorite. J Endod 36:725–728. https://doi.org/10.1016/j.joen.2009.11.017
Wang QQ, Zhang CF, Yin XZ (2007) Evaluation of the bactericidal effect of Er, Cr:YSGG, and Nd:YAG lasers in experimentally infected root canals. J Endod 33:830–832. https://doi.org/10.1016/j.joen.2007.03.017
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171. https://doi.org/10.1016/j.joen.2007.11.021
Widbiller M, Eidt A, Hiller KA, Buchalla W, Schmalz G, Galler KM (2017) Ultrasonic activation of irrigants increases growth factor release from human dentine. Clin Oral Investig 21:879–888. https://doi.org/10.1007/s00784-016-1824-1
de Groot SD, Verhaagen B, Versluis M, Wu MK, Wesselink PR, van der Sluis LW (2009) Laser-activated irrigation within root canals: cleaning efficacy and flow visualization. Int Endod J 42:1077–1083. https://doi.org/10.1111/j.1365-2591.2009.01634.x
Franzen R, Esteves-Oliveira M, Meister J, Wallerang A, Vanweersch L, Lampert F, Gutknecht N (2009) Decontamination of deep dentin by means of erbium, chromium:yttrium-scandium-gallium-garnet laser irradiation. Lasers Med Sci 24:75–80. https://doi.org/10.1007/s10103-007-0522-2
Azevedo LH, de Paula EF, Moreira MS, de Paula EC, Marques MM (2006) Influence of different power densities of LILT on cultured human fibroblast growth. Lasers Med Sci 21:86–89. https://doi.org/10.1007/s10103-006-0379-9
Breitbart H, Levinshal T, Cohen N, Friedmann H, Lubart R (1996) Changes in calcium transport in mammalian sperm mitochondria and plasma membrane irradiated at 633 nm (HeNe laser). J Photochem Photobiol B 34:117–121. https://doi.org/10.1016/1011-1344(95)07281-0
Yamakawa S, Niwa T, Karakida T, Kobayashi K, Yamamoto R, Chiba R, Yamakoshi Y, Hosoya N (2018) Effects of Er:YAG and diode laser irradiation on dental pulp cells and tissues. Int J Mol Sci 19(8):2429. https://doi.org/10.3390/ijms19082429
Ballini A, Mastrangelo F, Gastaldi G, Tettamanti L, Bukvic N, Cantore S, Cocco T, Saini R, Desiate A, Gherlone E, Scacco S (2015) Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: a good promise for tissue engineering. J Biol Regul Homeost Agents 29:813–822
Tabatabaei FS, Torshabi M, Nasab MM, Khosraviani K, Khojasteh A (2015) Effect of low-level diode laser on proliferation and osteogenic differentiation of dental pulp stem cells. Laser Physics 25:095602. https://doi.org/10.1088/1054-660x/25/9/095602
Acknowledgements
The authors thank Dr. M. Kevin O. Carroll for his assistance in the preparation of the manuscript and Dr. Thanapat Sastraruji for help in statistical analysis.
Funding
The work was supported by the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University and with the 1964 Helsinki declaration and its later amendments (No. 22/2017).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srisuwan, T., Srisathian, A. Improvement of apical papilla cell attachment after erbium, chromium-doped yttrium, scandium, gallium, and garnet laser application: a study in an ex vivo immature tooth model. Lasers Med Sci 37, 1167–1174 (2022). https://doi.org/10.1007/s10103-021-03368-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03368-0