Abstract
Following non-anatomical resection of lung parenchyma with a Nd:YAG laser, a coagulated surface remains. As ventilation starts, air leakage may occur in this area. The aim of the present study was to investigate, whether additional coagulation either before or after ventilation has an additional sealing effect. Freshly slaughtered porcine heart-lung blocks were prepared. The trachea was connected to a ventilator. Using a Nd:YAG laser (wavelength: 1320 nm, power: 60 W), round lesions (1.5 cm in diameter) with a depth of 1.5 cm were applied to the lung using an 800-μm laser fiber (5 s per lesion). Group 1 (n = 12) was control. Additional coagulation was performed in group 2 (n = 12) without and in group 3 (n = 12) with ventilation restarted. Air leakage (ml) from the lesions was measured. The thickness of each coagulation layer was determined on histological slices. Differences between individual groups were analyzed by one-way ANOVA (significance p < 0.05). After resection, 26.2 ± 2.7 ml of air emerged from the lesions per single respiration in group 1. Air loss in group 2 was 24.6 ± 2.5 ml (p = 0.07) and in group 3 23.7 ± 1.8 ml (p = 0.0098). In comparison to groups 1 and 2 thickness of the coagulation layers in group 3 was significantly increased. After non-anatomical porcine lung resection with a Nd:YAG laser, additional coagulation of the ventilated resection area can reduce air leakage.
Similar content being viewed by others
Introduction
Lung metastases are often removed non-anatomically using a Nd:YAG laser [1, 2], whereas pulmonary metastases are often removed via open access (thoracotomy). During open surgery the entire lung is carefully palpated. Following the technical trend minimally invasive procedures are increasingly favored, if technically feasible. In these procedures for non-anatomical resection of lung metastases thin laser fibers can be employed under thoracoscopic view [3]. After resection a coagulated resection surface remains bearing the risk of bleeding and air leakage. Therefore, in clinical routine frequently the resection area is additionally coagulated by laser. However, evidence regarding procedural risk or benefit of this procedure is scarce. In the center of a cutting lesion surgical lasers generate intense heat leading to tissue vaporization. However, near the margins of the vaporization zone the temperature is significantly lower and the lung tissue is merely coagulated. Depending on the energy of the laser, thickness of this coagulation zone may differ.
If coagulation of blood vessels is complete, a bloodless operating site may ensue [4, 5]. Especially in superficial resections [6] coagulation layers may well seal the lung parenchyma from air leaks. However, with increasing resection depth there is a growing risk of opening small bronchi or even segmental bronchi. The latter openings are clearly visible upon inspection of the resection surfaces and will lead to considerable air loss [6]. To avoid the risk of air leakage after laser resection in surgery, the majority of surgeons will routinely close resection sites by simple over-sutures. For deeper resections, several layers of sutures are used to avoid intrapulmonary cavitation and the potential risk of infection. On the other hand as a consequence of multiple laser resections and suturing, considerable, even functionally relevant restriction of the lung may develop [5]. Avoiding parenchymal suturing wherever possible therefore improves operative results.
Further sealing of resection surfaces by repeated laser coagulation is a possible option not yet sufficiently studied. The authors’ idea was that this will increase the thickness of an initial coagulation layer thereby improving airtightness.
In our study, we aimed at an experimental design to investigate the influence of lung ventilation during laser coagulation of pulmonary resection sites. In order to achieve good reproducibility, we opted for an ex-vivo model of porcine lungs.
Materials and methods
From freshly slaughtered pigs (weight 90 kg EU standard) the heart and both lungs were taken en bloc. As specimen were exclusively obtained from animals slaughtered for nutritional purposes, ACUC approval for animal studies or related body was not required.
The trachea was severed below the larynx. Remnants of the diaphragm and pericardium were removed. Preparations were examined for their external integrity and those with lesions on the lungs were discarded. Immediate transport to our laboratory followed. Only intact preparations were used for the experiments.
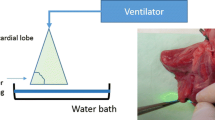
The trachea was intubated with a tube (Vygon 520, CH 8.0, Braun, Melsungen, Germany), and the tube balloon was blocked in the trachea. The tube was connected to a ventilator (Cicero EM PM 8060, Dräger, Lübeck, Germany), fixed to a board, and the entire preparation placed into a waterproof container. The intended lesions on the lung surface were marked by a stamp (diameter: 1.5 cm) and ventilation was stopped. All cylindrically shaped resections with a depth of 1.5 cm were performed with a Nd:YAG Laser LIMAX® 120 (wavelength: 1320 nm) (Gebrüder KLS Martin & Co., KG, Tuttlingen, Germany) and an 800-μm laser fiber at a laser power of 60 W (see Fig. 1a).
a Additional coagulation of the resection surface by laser fiber (power 60 W) in the ventilated lung. b Overview of the experimental setup. The ventilated lung is fixed by a grid. c To determine air leakage in ml/respiration, a funnel is pushed over the laser lesion. The ascending air (ml) is indicated on the volumetric flask
After resection, the lungs were ventilated. A peak inspiratory pressure of 25 mbar with a PEEP of + 5 mbar was chosen, this being equivalent to clinically employed pressures for determination of intraoperative air loss [7, 8]. Ventilation frequency was set to 10/min. To quantify air loss across the lesions, the lungs were submerged under water and held by a grid-like insert (see Fig. 1b). Lesions created by the laser were positioned underneath the grid openings. Air escaping from the lesions per single respirations was collected via a funnel filled with water attached to a volumetric flask (see Fig. 1c). Measurements were repeated five times for all lesions created and means were calculated.
We initially performed 12 resections without further intervention (group 1). In a second experiment additional coagulation of the lesions was performed with a laser power of 60 W at a distance of 1 cm to the lung surface for 5 s. Depending on the protocol, coagulation was carried out either before (group 2) or after renewed ventilation (group 3).
Tissue samples were taken from the resection areas for histological evaluation and sections were subjected to hematoxylin-eosin staining (HE). Thickness of coagulation zones (in μm) was measured using the program Image J version 1.46r (National Institutes of Health, Bethesda, USA).
Air losses of the individual groups were calculated via a one-way ANOVA and pairwise multiple comparison procedures (Holm–Sidak method). Statistical significance was set at p < 0.05. Regarding histological thickness of the coagulation layers groups were compared by non-parametric Mann Whitney U test (significance was set at p < 0.05).
The software package Graph Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis and creation of the graphs.
Results
In group 1 (no additional coagulation) an average air loss of 26.2 ± 2.7 ml per single respiration was determined. For this group the average thickness of the coagulation layer was determined to be 111.9 ± 1.5 μm. For group 2 (additional coagulation without ventilation) we calculated an air loss of 24.6 ± 2.5 ml after ventilation. Histological evaluation of the coagulation layer revealed an average thickness of 113.1 ± 1.4 μm. In group 3 (coagulation during renewed ventilation) mean air loss was 23.7 ± 1.8 ml. The histologically determined mean thickness of coagulation layers was 145.0 ± 2.2 μm (see Table 1 and Fig. 2). In all three groups the pattern of air leakage was observed in the form of continuous chains of bubbles.
Comparing the quantity of air lost, groups 1 and 2 did not differ significantly (p = 0.07). Similarly, there was no significant difference between groups 2 and 3 (p = 0.38) (see Tables 2 and 3). However, a significant difference was found between groups 1 and 3 (p = 0.0098). Looking at mean thicknesses of the coagulation layer, groups 1 and 2 were not significantly different (p = 0.06). In contrast, a highly significant difference (p < 0.0001) was found between groups 1 and 3 as well as between groups 2 and 3 (see Fig. 3).
Histological sections of the resection surfaces (HE staining). Arrows mark the margins of the coagulation zone. a Resection with laser power of 60 W. b Resection with laser fiber 60 W, then coagulation for 5 s at 60 W respire without ventilation. c Resection with laser fiber 60 W, then coagulation at 60 W for 5 s, ventilated lung. Magnification: 12.5-fold
Discussion
Airtightness after non-anatomical parenchymal lung resection with an Nd:YAG laser is a highly relevant clinical issue. Air leakage not only causes a prolonged chest drainage period but may also lead to longer inpatient treatment. Furthermore, in case of persistent air loss, operative revision of the leaking lung may be needed [9, 10]. Prolonged periods of chest tubes in situ in addition are associated with an increased risk of infection [9, 11]. Consequently, many thoracic surgeons always seal resection surfaces with sutures [12]. However, as additional suturing bears the risks of restriction and infection, effective coagulation may perhaps be the better alternative.
In a previous study [6] we were able to show that in superficial laser resections coagulation zones are sufficiently airtight. In deeper resections thickness of the coagulation layers are laser energy dependent [4].
If resection surfaces were coagulated in the ventilated lung, a significant decrease in the amount of air leaking was observed. The effect was clearly visible upon inspection and objectively confirmed by quantitative measurement. Resulting coagulation layers were increased in thickness. In theoretical thought ventilation of the resection surfaces will stretch the tissue so a potential for rupture of the primary coagulation layer may ensue. The previously resected and therefore partly coagulated parenchyma including potentially ruptured sites are additionally coagulated so that a more stable and thicker coagulation layer is produced. Branscheid [13] in 1992 reported a positive effect of coagulation of the resection surface after laser application on the half-distended lung. Additional laser coagulation after laser resection is a rather uncomplicated procedure, which, in clinical practice, is easily performed via an open or thoracoscopic access. Judging from our experimental findings, additional coagulation may be useful up to a resection depth of 1.5 cm. In deeper lesions, opening of small segmental bronchi is likely. According to our previous study [6], these bronchi are transected in the process of parenchymal resection and their lumina cannot be closed by coagulation. Suturing of the resection surfaces is required.
There are several limitations to this study. Heat generation in tissue by a Nd-YAG laser is physically based on exciting chromophores. Among chromophores involved are water, hemoglobin, and oxy-hemoglobin. In our experiments lungs were not perfused, thus reducing especially hemoglobin and oxy-hemoglobin, possibly influencing results. Furthermore, we only examined initial airtightness and we have no information regarding persistence of the effects stated over time. Therefore, as our investigations were performed ex vivo and neither on living tissue nor on human organs, we are not drawing clinical consequences.
Nevertheless, our experimental data contribute to the existing knowledge and we anticipate that they will facilitate current modeling and simulation efforts within the research group for laser-tissue interactions. As a possible next step an in vivo model may be suitable. Modifications of coagulation, e.g., duration of application and variations in the ventilation state of the lungs, should be further investigated
Conclusion
In an ex vivo porcine lung model, air loss via the resection surface after non-anatomical laser resection can be reduced by additional coagulation. Our data indicate that this should preferably be performed on the ventilated rather than on the non-ventilated lung.
References
Osei-Agyemang T et al (2013) Pulmonary metastasectomy: an analysis of technical and oncological outcomes in 301 patients with a focus on laser resection. Zentralbl Chir 138(Suppl 1):S45–S51
Franzke K et al (2017) Pulmonary metastasectomy: a retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur J Surg Oncol 43(7):1357–1364
Meyer C et al (2017) Video-assisted laser resection of lung metastases—feasibility of a new surgical technique. Thorac Cardiovasc Surg 65(5):382–386
Kirschbaum A et al (2012) Local effects of high-powered neodymium-doped yttrium aluminium garnet laser systems on the pulmonary parenchyma: an experimental study on the isolated perfused pig lung lobe. Interact Cardiovasc Thorac Surg 15(2):191–193
Rolle A et al (2002) Lobe-sparing resection of multiple pulmonary metastases with a new 1318-nm Nd:YAG laser—first 100 patients. Ann Thorac Surg 74(3):865–869
Kirschbaum A et al (2014) Airtightness of lung parenchyma without a closing suture after atypical resection using the Nd:YAG Laser LIMAX 120. Interact Cardiovasc Thorac Surg 18(1):92–95
Zaraca F et al (2017) Can a standardised Ventilation Mechanical Test for quantitative intraoperative air leak grading reduce the length of hospital stay after video-assisted thoracoscopic surgery lobectomy? J Vis Surg 3:179
Kim WH et al (2017) Intraoperative ventilatory leak predicts prolonged air leak after lung resection: a retrospective observational study. PLoS One 12(11):e0187598
Drahush N et al (2016) Standardized approach to prolonged air leak reduction after pulmonary resection. Ann Thorac Surg 101(6):2097–2101
Baringer K, Talbert S (2017) Chest drainage systems and management of air leaks after a pulmonary resection. J Thorac Dis 9(12):5399–5403
Pompili C, Miserocchi G (2016) Air leak after lung resection: pathophysiology and patients' implications. J Thorac Dis 8(Suppl 1):S46–S54
Venuta F et al (2010) Techniques used in lung metastasectomy. J Thorac Oncol 5(6 Suppl 2):S145–50.13
Branscheid D et al (1992) Does ND-YAG laser extend the indications for resection of pulmonary metastases? Eur J Cardiothorac Surg 6(11):590–596 discussion 597
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Andreas Kirschbaum is the first author with the following contributions: drafting of the manuscript, data collection, data analysis, critical revision of the manuscript; Thomas Surowiec contributed to the methodology, data analysis, manuscript editing, critical revision of the manuscript; Anika Pehl contributed to data analysis, methodology; Thomas Wiesmann contributed to experimental support, methodology, data collection; Detlef Bartsch contributed to editing and critical revision of the manuscript; Nikolas Mirow is the senior author and responsible for the design and conception, drafting of the manuscript, and critical revision with editing
Corresponding author
Ethics declarations
Ethical statement
An ethical vote was not needed, as the lung tissue used for the experiments originated from pigs slaughtered at a slaughterhouse for nutritional purposes.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kirschbaum, A., Surowiec, T.M., Pehl, A. et al. Local lung coagulation post resection: an ex-vivo porcine model. Lasers Med Sci 37, 443–447 (2022). https://doi.org/10.1007/s10103-021-03280-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03280-7