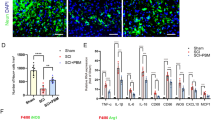
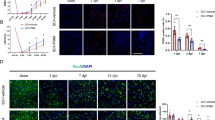
Abstract
In spinal cord injury (SCI), inflammation is a major mediator of damage and loss of function and is regulated primarily by the bone marrow-derived macrophages (BMDMs). Photobiomodulation (PBM) or low-level light stimulation is known to have anti-inflammatory effects and has previously been used in the treatment of SCI, although its precise cellular mechanisms remain unclear. In the present study, the effect of PBM at 810 nm on classically activated BMDMs was evaluated to investigate the mechanisms underlying its anti-inflammatory effects. BMDMs were cultured and irradiated (810 nm, 2 mW/cm2) following stimulation with lipopolysaccharide and interferon-γ. CCK-8 assay, 2′,7′-dichlorofluorescein diacetate assay, and ELISA and western blot analysis were performed to measure cell viability, reactive oxygen species production, and inflammatory marker production, respectively. PBM irradiation of classically activated macrophages significantly increased the cell viability and inhibited reactive oxygen species generation. PBM suppressed the expression of a marker of classically activated macrophages, inducible nitric oxide synthase; decreased the mRNA expression and secretion of pro-inflammatory cytokines, tumor necrosis factor alpha, and interleukin-1 beta; and increased the secretion of monocyte chemotactic protein 1. Exposure to PBM likewise significantly reduced the expression and phosphorylation of NF-κB p65 in classically activated BMDMs. Taken together, these results suggest that PBM can successfully modulate inflammation and polarization in classically activated BMDMs. The present study provides a theoretical basis to support wider clinical application of PBM in the treatment of SCI.






Similar content being viewed by others
References
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 71(2):281–299
Faden AI, Wu J, Stoica BA et al (2016) Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol 173(4):681–691
Horn KP, Busch SA, Hawthorne AL et al (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28(38):9330–9341
Busch SA, Horn KP, Silver DJ et al (2009) Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci 29(32):9967–9976
Busch SA, Hamilton J, Horn KP et al (2011) Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci 31(3):944–953
Nordendiana M, Fawtimothy D, Mckimdaniel B et al (2018) Bone marrow-derived monocytes drive the inflammatory microenvironment in local and remote regions after thoracic spinal cord injury. J Neurotrauma 2019,36(6):1–37
Greenhalgh AD, David S (2014) Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci 34(18):6316–6322
Evans TA, Barkauskas DS, Myers JT et al (2014) High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol 254(4):109–120
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Kigerl KA, Gensel JC, Ankeny DP et al (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29(43):13435–13444
Ren Y, Young W (2013) Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast 2013:945034
Samuel D, Antje K (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399
Guerrero AR, Uchida K, Nakajima H et al (2012) Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation 9(1):40
Li F, Cheng B, Cheng J et al (2015) CCR5 blockade promotes M2 macrophage activation and improves locomotor recovery after spinal cord injury in mice. Inflammation 38(1):126–133
Ji XC, Dang YY, Gao HY et al (2015) Local injection of Lenti–BDNF at the lesion site promotes M2 macrophage polarization and inhibits inflammatory response after spinal cord injury in mice. Cell Mol Neurobiol 35(6):881–890
Zhang Q, Bian G, Chen P et al (2014) Aldose reductase regulates microglia/macrophages polarization through the cAMP response element-binding protein after spinal cord injury in mice. Mol Neurobiol 53(1):662–676
T D, SK S, YY H et al (2012) The nuts and bolts of low-level laser (light) therapy.%A Chung H. Ann Biomed Eng 40(2):516–533
Manstein D, Laubach H, Watanabe K et al (2008) Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med 40(9):595–604
Jori G, Fabris C, Soncin M et al (2010) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38(5):468–481
Jimenez JJ, Wikramanayake TC, Bergfeld W et al (2014) Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol 15(2):115–127
Lívia A, Moretti AIS, Abrah OTB et al (2012) Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg Med 44(9):726–735
Byrnes KR, Waynant RW, Ilev IK et al (2005) Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 36(3):171–185
Wu X, Dmitriev AE, Cardoso MJ et al (2009) 810 nm wavelength light: an effective therapy for transected or contused rat spinal cord. Lasers Surg Med 41(1):36–41
Hu D, Zhu S, Potas JR (2016) Red LED photobiomodulation reduces pain hypersensitivity and improves sensorimotor function following mild T10 hemicontusion spinal cord injury. J Neuroinflammation 13(1):200
Hu D, Zhu S, Potas JR (2016) Red LED photobiomodulation reduces pain hypersensitivity and improves sensorimotor function following mild T10 hemicontusion spinal cord injury. J Neuroinflammation 13(1):200
Song JW, Li K, Liang ZW et al (2017) Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci Rep 7(1):620
Gavish L, Perez LS, Reissman P et al (2008) Irradiation with 780 nm diode laser attenuates inflammatory cytokines while upregulating nitric oxide in LPS-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg Med 40(5):371–378
Souza NHC, Marcondes PT, Albertini R et al (2014) Low-level laser therapy suppresses the oxidative stress-induced glucocorticoids resistance in U937 cells: relevance to cytokine secretion and histone deacetylase in alveolar macrophages. J Photochem Photobiol B Biol 130(1):327–336
Ki Bum A, Seok-Seong K, Ok-Jin P et al (2014) Irradiation by gallium-aluminum-arsenate diode laser enhances the induction of nitric oxide by Porphyromonas gingivalis in RAW 264.7 cells. J Periodontol 85(9):1259–1265
Fernandes KPS, Souza NHC, Mesquita-Ferrari RA et al (2015) Photobiomodulation with 660-nm and 780-nm laser on activated J774 macrophage-like cells: effect on M1 inflammatory markers. J Photochem Photobiol B Biol 153:344–351
Leden REV, Cooney SJ, Ferrara TM et al (2013) 808?nm wavelength light induces a dose-dependent alteration in microglial polarization and resultant microglial induced neurite growth. Lasers Surg Med 45(4):253–263
Meerpohl HG, Lohmann-Matthes ML, Fischer H (2010) Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol 6(3):213–217
Kigerl KA, Gensel JC, Ankeny DP et al (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29(43):13435–13444
Hamblin MR, Huang YY, Heiskanen V (2019) Non-mammalian hosts and photobiomodulation: do all life-forms respond to light? 95(1):126–Photochem Photobiol, 139
Leung MCP, Lo SCL, Siu FKW et al (2010) Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med 31(4):283–288
Silva IHM, Andrade SCD, Fonsêca DDD et al (2016) Increase in the nitric oxide release without changes in cell viability of macrophages after laser therapy with 660 and 808 nm lasers. Lasers Med Sci 31(9):1855–1862
Elena SF, Concha N, Angeles DS et al (2014) CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 192(8):3858–3867
Jung KM, Hae Young S, Yuexian C et al (2015) CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci 35(48):15934–15947
Alexandratou E, Yova D, Handris P et al (2002) Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy. Photochem Photobiol Sci 1(8):547–552
Hume DA (2015) The many alternative faces of macrophage activation. Front Immunol 6:1–10
Yu XJ, Zhang DM, Jia LL et al (2015) Inhibition of NF-κB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol Appl Pharmacol 284(3):315–322
Jurk D, Wilson CL, Passos JF et al (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 5(1):4172
Chung IS, Kim JA, Kim JA et al (2013) Reactive oxygen species by isoflurane mediates inhibition of nuclear factor κB activation in lipopolysaccharide-induced acute inflammation of the lung. Anesth Analg 116(2):327–335
Acknowledgments
We thank Xi’an laser tech medical technology company LTD for providing the PBM device. The authors also wish to thank Prof. Jielai Xia from AFMU, China, for editing this manuscript.
Funding
This work is supported by grants from the National Natural Science Foundation of China (No. 81572151) and Key Science and Technology Program in Social Development of Shaanxi Province (2016SF-143).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All the procedures that required the use of mice were performed following the guidelines established by the Animal Care Ethics Committee of the Fourth Military Medical University, Xi’an, P.R. China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhe Wang is corresponding author and Xueyu Hu is the co-corresponding author.
Kun Li, Zhuowen Liang and Jiawei Zhang are co-first author.
Rights and permissions
About this article
Cite this article
Li, K., Liang, Z., Zhang, J. et al. Attenuation of the inflammatory response and polarization of macrophages by photobiomodulation. Lasers Med Sci 35, 1509–1518 (2020). https://doi.org/10.1007/s10103-019-02941-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02941-y