Abstract
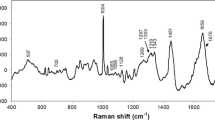
Urea and creatinine are commonly used as biomarkers of renal function. Abnormal concentrations of these biomarkers are indicative of pathological processes such as renal failure. This study aimed to develop a model based on Raman spectroscopy to estimate the concentration values of urea and creatinine in human serum. Blood sera from 55 clinically normal subjects and 47 patients with chronic kidney disease undergoing dialysis were collected, and concentrations of urea and creatinine were determined by spectrophotometric methods. A Raman spectrum was obtained with a high-resolution dispersive Raman spectrometer (830 nm). A spectral model was developed based on partial least squares (PLS), where the concentrations of urea and creatinine were correlated with the Raman features. Principal components analysis (PCA) was used to discriminate dialysis patients from normal subjects. The PLS model showed r = 0.97 and r = 0.93 for urea and creatinine, respectively. The root mean square errors of cross-validation (RMSECV) for the model were 17.6 and 1.94 mg/dL, respectively. PCA showed high discrimination between dialysis and normality (95 % accuracy). The Raman technique was able to determine the concentrations with low error and to discriminate dialysis from normal subjects, consistent with a rapid and low-cost test.




Similar content being viewed by others
References
Alpern RJ, Caplan MJ, Moe OW (2013) Seldin and Giebisch’s the kidney—physiology and pathophysiology, vol 1. Academic Press, Waltham
About chronic kidney disease: a guide for patients and their families (2010) National Kidney Foundation. http://www.kidney.org/atoz/atozcopy.cfm?pdflink=11-50-0160_jai_patbro_aboutckdv2lr.pdf. Accessed 10 Mar 2014.
Bastos MG, Bregman R, Kirsztajn GM (2010) Chronic kidney diseases: common and harmful, but also preventable and treatable. Rev Assoc Med Bras 56:248–53
Von Schacky C (2009) Cardiovascular disease prevention and treatment. Prostaglandins Leukot Essent Fatty Acids 81(2-3):193–8
Centers for Disease Control (CDC) (1992) Incidence of treatment for end-stage renal disease attributed to diabetes mellitus, by race/ethnicity-Colorado, 1982-1989. Morb Mortal Wkly Rep 41(44):845–8
Haase-Fielitz A, Bellomo R, Devarajan P et al (2009) Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—a prospective cohort study. Crit Care Med 37(2):553–60
Gabriel IC, Nishidal SK, Kirsztajn GM (2011) Serum cystatin C: a practical alternative for renal function evaluation? J Bras Nefrol 33:261–7
Perez-Guaita D, Ventura-Gayete P-RC, Sancho-Andreu M, Garrigues S, de la Guardia M (2013) Evaluation of infrared spectroscopy as a screening tool for serum analysis: impact of the nature of samples included in the calibration set. Microchem J 106:202–11
Prat R, Galeano I, Lucas A et al (2010) Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci 17(1):50–3
Pilotto S, Pacheco MTT, Silveira L, Villaverde AB, Zângaro RA (2001) Analysis of near-infrared Raman spectroscopy as a new technique for a transcutaneous non-invasive diagnosis of blood components. Lasers Med Sci 16(1):2–9
Rohleder D, Kocherscheidt G, Gerber K et al (2005) Comparison of mid-infrared and Raman spectroscopy in the quantitative analysis of serum. J Biomed Opt 10(3):031108
Chaiken J, Finney WF, Knudson PE et al (2001) Noninvasive in-vivo tissue-modulated Raman spectroscopy of human blood: microcirculation and viscosity effects. Proc. SPIE 4254, Biomedical Diagnostic, Guidance, and Surgical-Assist Systems III, 106. doi: 10.1117/12.427952.
Berger AJ, Koo TW, Itzkan I, Horowitz G, Feld MS (1999) Multicomponent blood analysis by near-infrared Raman spectroscopy. Appl Opt 38(13):2916–26
Rohleder D, Kiefer W, Petrich W (2004) Quantitative analysis of serum and serum ultrafiltrate by means of Raman spectroscopy. Analyst 129(10):906–11
Qi D, Berger AJ (2007) Chemical concentration measurement in blood serum and urine samples using liquid-core optical fiber Raman spectroscopy. Appl Opt 46(10):1726–34
Hanlon EB, Manoharan R, Koo TW et al (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45(2):R1–59
González-Solís JL, Martínez-Espinosa JC, Torres-González LA, Aguilar-Lemarroy A, Jave-Suárez LF, Palomares-Anda P (2014) Cervical cancer detection based on serum sample Raman spectroscopy. Lasers Med Sci 29(3):979–85
Premasiri WR, Clarke RH, Womble ME (2001) Urine analysis by laser Raman spectroscopy. Laser Surg Med 28:330–4
Smith E, Dent G (2005) Modern Raman spectroscopy: a practical approach. John Wiley & Sons, Chiclester
Guimarães AE, Pacheco MTT, Silveira L et al (2006) Near infrared Raman spectroscopy (NIRS): a technique for doping control. Spectrosc Int J 20:185–94
Silveira L, Sathaiah S, Zângaro RA, Pacheco MT, Chavantes MC, Pasqualucci CA (2002) Correlation between near-infrared Raman spectroscopy and the histopathological analysis of atherosclerosis in human coronary arteries. Laser Surg Med 30:290–7
Bispo JAM, de Sousa Vieira EE, Silveira L, Fernandes AB (2013) Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J Biomed Opt 18(8):87004. doi:10.1117/1.JBO.18.8.087004
Enejder AM, Koo TW, Oh J et al (2002) Blood analysis by Raman spectroscopy. Opt Lett 27:2004–6
Saade J, Pacheco MTT, Rodrigues MR, Silveira L (2008) Identification of hepatitis C in human blood serum by near-infrared Raman spectroscopy. Spectroscopy Int J 22:387–95. doi:10.3233/SPE-2008-0344
Ito H, Inoue H, Hasegawa K et al (2014) Use of surface-enhanced Raman scattering for detection of cancer-related serum-constituents in gastrointestinal cancer patients. Nanomedicine 10(3):599–608
Chatfield C, Collins AJ (1980) Introduction to multivariate statistics. Chapman & Hall, London
Bueno Filho AC, Silveira L, Yanai ALS, Fernandes AB (2015) Raman spectroscopy for a rapid diagnosis of sickle cell disease in human blood samples: a preliminary study. Lasers Med Sci 30(1):247–53
Bodanese B, Silveira FL, Zângaro RA, Pacheco MTT, Pasqualucci CA, Silveira L (2012) Discrimination of basal cell carcinoma and melanoma from normal skin biopsies in vitro through Raman spectroscopy and principal component analysis. Photomed Laser Surg 30(7):381–7
Lui H, Zhao J, McLean D, Zeng H (2012) Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res 72(10):2491–500
Silveira FL, Pacheco MTT, Bodanese B, Pasqualucci CA, Zângaro RA, Silveira L (2015) Discrimination of non-melanoma skin lesions from non-tumor human skin tissues in vivo using Raman spectroscopy and multivariate statistics. Lasers Surg Med 47(1):6–16
Chowdary MV, Kumar KK, Kurien J, Mathew S, Krishna CM (2006) Discrimination of normal, benign, and malignant breast tissues by Raman spectroscopy. Biopolymers 83(5):556–69
Biosystems S/A (2011) UREA/BUN - UV (Urease/Glutamato Deshidrogenase). http://www.imunotech.com.br/bulas/ureia.pdf. Accessed 12 Mar 2014.
Eurotech Prod. Laboratoriais e Serviços (2012) Creatinina “Mod. Jaffe”. Reagente diagnóstico para determinação quantitativa in vitro da creatinina em soro humano, plasma ou urina em sistemas fotométricos. http://www.imunotech.com.br/bulas/rea_5073240e63694.pdf. Accessed 12 Mar 2014.
Silveira L, Silveira FL, Bodanese B, Zângaro RA, Pacheco MT (2012) Discriminating model for diagnosis of basal cell carcinoma and melanoma in vitro based on the Raman spectra of selected biochemicals. J Biomed Opt 17:077003
Martins JPA, Ferreira M (2013) QSAR modeling: a new open source computational package to generate and validate QSAR model. Quim Nov. 36(4):554–60. doi:10.1590/S0100-40422013000400013
Geladi P, Kowalski BR (1986) Partial least-squares regression: a tutorial. Anal Chim Acta 185:1–17
Brereton RG (2000) Introduction to multivariate calibration in analytical chemistry. Analyst 125(11):2125–54
Nurunnabi AAM, Hadi AS, Imon AHMR (2014) Procedures for the identification of multiple influential observations in linear regression. J Appl Stat 41(6):1315–31
Black PE (2004) Euclidean Distance. U.S. National Institute of Standards and Technology. http://www.nist.gov/dads/HTML/euclidndstnc.html. Accessed 07 May 2014.
Ivanov AI, Zhbankov RG, Korolenko EA et al (1994) Infrared and Raman spectroscopic studies of the structure of human serum albumin under various ligand loads. J Appl Spectrosc 60(5-6):305–9
Saha A, Yakovlev VV (2010) Structural changes of human serum albumin in response to a low concentration of heavy ions. J Biophotonics 3(10-11):670–7
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 42(5):493–541
Bayrak C, Bayari SH (2010) Vibrational and DFT studies of creatinine and its metal complexes. Hacett J Biol Chem 38(2):107–18
Keuleers R, Desseyn HO, Rousseau B, Van Alsenoy C (1999) Vibrational analysis of urea. J Phys Chem 103(24):4621–30
Pichardo-Molina JL, Frausto-Reyes C, Barbosa-García O et al (2007) Raman spectroscopy and multivariate analysis of serum samples from breast cancer patients. Lasers Med Sci 22(4):229–36
Stosch R, Henrion A, Schiel D, Güttler B (2005) Surface-enhanced Raman scattering based approach for quantitative determination of creatinine in human serum. Anal Chem 77:7386–92
Peake M, Whiting M (2006) Measurement of serum creatinine—current status and future goals. Clin Biochem Rev 27(4):173–84
Weykamp C, Kuypers A, Bakkeren D, Franck P, van Loon D, Klein Gunnewiek J, de Jonge R, Steigstra H, Cobbaert C (2015) Creatinine, Jaffe, and glucose: another inconvenient truth. Clin Chem Lab Med 53(12):e347–9
Panteghini M (2008) Enzymatic assays for creatinine: time for action. Clin Chem Lab Med 46(4):567–72
Acknowledgments
L. Silveira Jr. thanks São Paulo Research Foundation (FAPESP) for supporting the acquisition of the Raman spectrometer (Process no. 2009/01788-5). C. J. Saatkamp and M. L. Almeida thank Fundação Esperança, sponsor of the Instituto Esperança de Educação Superior (IESPES) for partly funding this research (Process no. 05/2012). The authors acknowledge the support of the Laboratório Celso Matos in this survey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Almeida, M.L., Saatkamp, C.J., Fernandes, A.B. et al. Estimating the concentration of urea and creatinine in the human serum of normal and dialysis patients through Raman spectroscopy. Lasers Med Sci 31, 1415–1423 (2016). https://doi.org/10.1007/s10103-016-2003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2003-y