Abstract
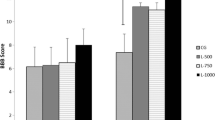
Scientific advances have been made to optimize the healing process in spinal cord injury. Studies have been developed to obtain effective treatments in controlling the secondary injury that occurs after spinal cord injury, which substantially changes the prognosis. Low-intensity laser therapy (LILT) has been applied in neuroscience due to its anti-inflammatory effects on biological tissue in the repairing process. Few studies have been made associating LILT to the spinal cord injury. The objective of this study was to investigate the effect of the LILT (GaAlAs laser—780 nm) on the locomotor functional recovery, histomorphometric, and histopathological changes of the spinal cord after moderate traumatic injury in rats (spinal cord injury at T9 and T10). Thirty-one adult Wistar rats were used, which were divided into seven groups: control without surgery (n = 3), control surgery (n = 3), laser 6 h after surgery (n = 5), laser 48 h after surgery (n = 5), medullar lesion (n = 5) without phototherapy, medullar lesion + laser 6 h after surgery (n = 5), and medullar lesion + laser 48 h after surgery (n = 5). The assessment of the motor function was performed using Basso, Beattie, and Bresnahan (BBB) scale and adapted Sciatic Functional Index (aSFI). The assessment of urinary dysfunction was clinically performed. After 21 days postoperative, the animals were euthanized for histological and histomorphometric analysis of the spinal cord. The results showed faster motor evolution in rats with spinal contusion treated with LILT, maintenance of the effectiveness of the urinary system, and preservation of nerve tissue in the lesion area, with a notorious inflammation control and increased number of nerve cells and connections. In conclusion, positive effects on spinal cord recovery after moderate traumatic spinal cord injury were shown after LILT.





Similar content being viewed by others
Abbreviations
- Group C:
-
Control without surgery (n = 3)
- Group CC:
-
Control surgery, only incision and exposition of vertebral column (n = 3)
- Group ML:
-
Medullar lesion without phototherapy (n = 5)
- Group LA:
-
Laser 6 h after surgery, incision and exposition of vertebral column, and irradiation (n = 5)
- Group LB:
-
Laser 48 h after surgery, incision and exposition of vertebral column, and irradiation (n = 5)
- Group MLA:
-
Medullar lesion + laser 6 h after surgery (n = 5)
- Group MLB:
-
Medullar lesion + laser 48 h after surgery (n = 5)
References
Vall J, Braga VAB, Almeida PC (2006) Study of the quality of life in people with traumatic spinal cord injury. Arq Neuropsiquiatr 64(2b):451–455. doi:10.1590/S0004-282X2006000300019
Albuquerque ALP, Freitas CHA, Jorge MSB (2009) Interpreting the hospitalization experiences of patients with spine lesion. Rev Bras Enferm 62(4):552–556. doi:10.1590/S0034-71672009000400010
Molina AEIS, Barros Filho TEP (2004) Comparative analysis of functional evaluation performed in medullary injury in animals. Acta Ortop Bras 2:35–46. doi:10.1590/S1413-78522004000100007
Scivoletto G et al (2007) Plasticity of spinal centers in spinal cord injury patients: new concepts for gait evaluation and training. Neurorehabil Neural Repair 21(4):358–365. doi:10.1177/1545968306295561
Young W (1993) Secondary injury mechanisms in acute spinal cord injury. J Emerg Med 11(1):13–22
Dusart I, Schuwab ME (1994) Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci 6(5):712–724. doi:10.1111/j.1460-9568
Tator CH, Fehlings MG (1991) Review of secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanism. J Neurosurg 75(1):15–26. doi:10.3171/jns.1991.75.1.0015
McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW (1999) Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5(14):10–12. doi:10.1038/70986
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? J Neurosci 27(44):11782–11792. doi:10.1523/3444-07.2007
dos Reis FA, Belchior AC, de CarvalhoPde T, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2008) Effect of gallium-aluminum-arsenide laser therapy (660 Nm) on recovery of the sciatic nerve in rats following neurotmesis lesion and epineural anastomosis: functional analysis. Rev Bras Fisioter 12:215–221. doi:10.1590/S1413-35552008000300009
Belchior AC, dos Reis FA, Nicolau RA, Silva IS, Perreira DM, de CarvalhoPde T (2009) Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24(6):893–899. doi:10.1007/s10103-008-0642-3
Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ (2005) Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 36:171–185. doi:10.1002/lsm.20143
Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR (2007) Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 39(9):706–715. doi:10.1002/lsm.20549
dos Reis FA, Belchior AC, de CarvalhoPde T, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747. doi:10.1007/s10103-008-0634-3
Piva JAAC, Abreu EMC, Silva VS, Nicolau RA (2011) Effect of low-level laser therapy on the initial stages of tissue repair: basic principles. An Bras Dermatol 86(5):947–954. doi:10.1590/S0365-05962011000500013
Alves AC, de Carvalho PT, Parente M, Xavier M, Frigo L, Aimbire F, Leal Junior EC, Albertini R (2013) Low-level laser therapy in different stages of rheumatoid arthritis: a histological study. Lasers Med Sci 28(2):529–536. doi:10.1007/s10103-012-1102-7
Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J, Anders JJ (2009) 810 nm wavelength light: an effective therapy for transected or contused rat spinal cord. Lasers Surg Med 41:36–41. doi:10.1002/lsm.20729
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533. doi:10.1007/s10439-011-0454-7
von Leden RE, Cooney SJ, Ferrara TM, Zhao Y, Dalgard CL, Anders JJ, Byrnes KR (2013) 808 nm wavelength light induces a dose-dependent alteration in microglial polarization and resultant microglial induced neurite growth. Lasers Surg Med 45:253–263. doi:10.1002/lsm.22133
Ando T, Sato S, Kobayashi H, Nawashiro H, Ashida H, Hamblin MR, Obara M (2013) Low-level laser therapy for spinal cord injury in rats: effects of polarization. J Biomed Opt 18(9):098002. doi:10.1117/1.JBO.18.9.098002
Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y et al (2013) Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One 8(1):e53454. doi:10.1371/journal.pone.0053454
Touitou Y, Portaluppi F, Smolensky MH, Rensing L (2004) Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol Int 21(1):161–170. doi:10.1081/CBI-120030045
Andersen ML, D’Almeida V, Ko GM et al (2004) Ethical and practical principles of the use of experimental animals. UNIFESP—Universidade Federal de São Paulo, São Paulo
Young W (2002) Spinal cord contusion models. Prog Brain Res 137:231–255. doi:10.1007/978-1-60327-185-1_35
Dombourian MG, Turner NA, Gerovac TA, Vemuganti R, Miranpuri GS, Tureyen K et al (2006) B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine (Phila Pa 1976).31(24):2778-2782 doi: DOI:10.1097/01.brs.0000245865.97424.b4
Onifer SM, Rabchevsky AG, Scheff SW (2007) Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J 48(4):385–395. doi:10.1093/ilar.48.4.385
Pinzon A et al (2008) A Re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res 1243:146–151. doi:10.1016/j.brainres.2008.09.047
Rodrigues NR, Letaif OB, Cristante AF, Marcon RM, Oliveira RP, Barros Filho TEP (2010) Standardization of spinal cord injury in Wistar rats. Acta Ortop Bras 18(4):182–186. doi:10.1590/S1413-78522010000400001
Santos GB, Cristante AF, Marcon RM, Souza FI, Barros Filho TEP, Damasceno ML (2011) Spinal cord injury experimental model and motion evaluation protocol in Wistar rats. Actaortop bras 19(2):87–91. doi:10.1590/S1413-78522011000200005
Vijayaprakash KM, Sridharan N (2013) An experimental spinal cord injury rat model using customized impact device: a cost-effective approach. J Pharmacol Pharmacother 4(3):211–213. doi:10.4103/0976-500X.114607
Barros Filho TEP, Molina AEIS (2008) Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics 63(1):103–108. doi:10.1590/S1807-59322008000100018
De Medinacelli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77:634–643. doi:10.1016/0014-4886(82)90234-5
Varejão AS, Cabrita AM, Geuna S, Melo-Pinto P, Filipe VM, Gramsbergen A, Meek MF (2003) Toe out angle: a functional index for the evaluation of sciatic nerve recovery in the rat model. Exp Neurol 183:695–699. doi:10.1016/S0014-4886(03) 00208-5
Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A (2000) Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods 96:89–96. doi:10.1016/S0165-0270(99)00174-0
Nash HH, Borke RC, Anders JJ (2002) Ensheathing cells and methylprednisolone promote axonal regeneration and functional recovery in the lesioned adult rat spinal cord. J Neurosci 2216:7111–7120. doi:10.1016/j.nbd.2008.09.012
García-Alías G, López-Vales R, Forés J, Navarro X, Verdú E (2004) Acute transplantation of olfactory ensheathing cells or Schwann cells promotes recovery after spinal cord injury in the rat. J Neurosci Res 75(5):632–641. doi:10.1002/jnr.20029
Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B (2008) Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor1 (IGF1), and receptor of IGF1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci 23(2):211–215. doi:10.1007/s10103-007-0477-3
Albertini R, Aimbire F, Villaverde AB, Silva JA Jr, Costa MS (2007) COX-2 mRNA expression decreases in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low level laser therapy. Inflamm Res 56:228–229. doi:10.1007/s00011-007-6211-6
Albertini R, Aimbire FS, Correa FI, Ribeiro W, Cogo JC, Antunes E, Teixeira SA, De Nucci G, Castro-Faria-Neto HC, Zângaro RA, Lopes-Martins RA (2004) Effects of different protocol doses of low power gallium-aluminum-arsenate (Ga-Al-As) laser radiation (650 nm) on carrageenan induced rat paw oedema. J Photochem Photobiol B 74:101–107. doi:10.1016/j.jphotobiol.2004.03.002
Mizutani K, Musya Y, Wakae K, Kobayashi T, Tobe M, Taira K, Harada T (2004) A clinical study on serum prostaglandin E2 with lowlevel laser therapy. Photomed Laser Surg 22(6):537–539. doi:10.1089/pho.2004.22.537
Andrade MS, Hanania FR, Daci K, Leme RJ, Chadi G (2008) Contuse lesion of the rat spinal cord of moderate intensity leads to a higher time-dependent secondary neurodegeneration than severe one an open-window for experimental neuroprotective interventions. Tissue Cell 40:143–156. doi:10.1016/j.tice.2007.11.002
Beattie MS, Farooqui AA, BRESNAHAN JC (2000) Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma 17:915–925. doi:10.1089/neu.2000.17.915
Streit WJ (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. GLIA 40:133–139. doi:10.1002/glia.10154
Leme RJ, Chadi G (2001) Distant microglial and astroglial activation secondary to experimental spinal cord lesion. Arq Neuropsiquiatr 59:483–492. doi:10.1590/S0004-282X2001000400002
Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT (2005) Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 280:4761–4771. doi:10.1074/jbc.M409650200
Lubart R, Breitbart H (2000) Biostimulative effects of low energy lasers and their implications for medicine. Drug Dev Res 50:471–475. doi:10.1002/1098-2299
Pineau I, Lacroix S (2007) Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500:267–285. doi:10.1002/cne.21149
Nomura K, Yamaguchi M, Abiko Y (2001) Inhibition of interleukin-1beta production and gene expression in human gingival fibroblasts by low-energy laser irradiation. Lasers Med Sci 16:218–223. doi:10.1007/PL00011358
Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM (2002) The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol 61(7):623–633
Aimbire F, Albertini R, Pacheco MT, Castro-Faria-Neto HC, Leonardo PS, Iversen VV, Lopes-Martins RA, Bjordal JM (2006) Low level laser therapy induces dose dependent reduction of TNFα levels in acute inflammation. Photomed Laser Surg 24(1):33–37. doi:10.1089/pho.2006.24.33
Meyer F, Vialle LR, Vialle EM, Bleggi-Torres LF, Rasera E, Leonel I (2003) Urinary bladder changes in experimental medullary lesion in rats. Acta Cir Bras 18(3):203–207. doi:10.1590/S0102-86502003000300007
Siroky MB (2002) Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 113(Suppl 1A):67S–79S. doi:10.1016/S0002-9343(02)01061-6
Krassioukov A, Darren EW, Teassel R, Eng JJ (2009) A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 90(4):682–695. doi:10.1016/j.apmr.2008.10.017
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paula, A.A., Nicolau, R.A., Lima, M.d.O. et al. “Low-intensity laser therapy effect on the recovery of traumatic spinal cord injury”. Lasers Med Sci 29, 1849–1859 (2014). https://doi.org/10.1007/s10103-014-1586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1586-4