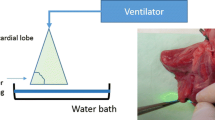
Abstract
Laser-directed resection of lung metastases is performed more frequently in recent years. The energy-loaded laser rays heat up the lung tissue, considerably. It is still unclear which mechanism is more important for tissue heat dissipation: the lung perfusion or the tissue emission. Therefore, we created a special experimental model to investigate the spontaneous heat dissipation after nonanatomical lung resection using a diode-pumped laser with a high output power. Experiments were conducted on paracardiac pig lung lobes (n = 12) freshly dissected at the slaughterhouse. Nonanatomical resection of lung parenchyma was performed without lobe perfusion in group 1 (n = 6), while group 2 (n = 6) was perfused at a physiological pressure of 25 cm H2O at 37 °C with saline via the pulmonary artery. For this, we used a diode-pumped neodymium-doped yttrium aluminum garnet (Nd:YAG) LIMAX® 120 laser (Gebrüder Martin GmbH & Co. KG, Tuttlingen, Germany) with a wavelength of 1,318 nm and a power output of 100 W. Immediately after completing laser resection, the lungs were monitored with an infrared camera (Type IC 120LV; Trotec, Heinsberg, Germany) while allowed to cool down. The resection surface temperature was taken at 10-s intervals and documented in a freeze-frame until a temperature of 37 °C had been reached. The temperature drop per time unit was analyzed in both groups. Immediately after laser resection, the temperature at the lung surface was 84.33 ± 8.08 °C in group 1 and 76.75 ± 5.33 °C in group 2 (p = 0.29). Group 1 attained the final temperature of 37 °C after 182.95 ± 53.76 s, and group 2 after 121.70 ± 16.02 s (p = 0.01). The temperature drop occurred exponentially in both groups. We calculated both groups’ decays using nonlinear regression, which revealed nearly identical courses. The mean time of tissue temperature of >42 °C, as a surrogate marker for tissue damage, was 97.14 ± 26.90 s in group 1 and 65.00 ± 13.78 s in group 2 (p = 0.02). Heat emission to the environment surpasses heat reduction via perfusion in nonanatomically laser-resected lung lobes. In developing a cooling strategy, a topical cooling method would be promising.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
About 30 % of all tumor patients develop lung metastases [1]. These can occur uni- or bilaterally, solitary, or multiple. Physicians should discuss the surgical option of lung metastasis removal with patients presenting a favorable prognosis, such as R0 status, no other distant metastases, and adequate cardiopulmonary capacity [2]. Laser-assisted resection has been more widely performed in recent years in addition to conventional resection techniques [3, 4]. The laser technology enables the surgeon to remove numerous metastases of various sizes with minimal blood loss in one intervention, while sparing lung parenchyma compared to conventional resection techniques [5]. In the past, surgeons worked with a laser power output of 60 W or even lower; however, to increase resection effectiveness and decrease surgery times, we decided to work with one of the novel high-power laser devices in the future. One of the advanced lasers is the diode-pumped neodymium-doped yttrium aluminum garnet (Nd:YAG) LIMAX® 120 laser (KLS Martin, Tuttlingen, Germany), which provides a maximum laser output of 120 W. Lenses concentrate the laser beam, which hits the lung parenchyma at a wavelength of 1,318 nm. Its maximum output creates >170 kW/cm2 onto areas as small as 260 μm2. This energy is absorbed by the tissue and converted into heat. A focused laser beam can raise the tissue temperature to approx. 800 °C, causing it to vaporize, whereas the tissue coagulates at 60 to 100 °C in the resection margins [6]. Temperatures of 30 to 60 °C are reached in the areas surrounding the coagulation zones, and thermal–chemical processes can be triggered in those cells. Temperatures maintained above 45 °C for a prolonged period of time cause irreversible cell damage. Between 50 and 52 °C, the time to cytotoxicity is reduced to 4 to 6 min [7–9]. Genuine cell damage is difficult to diagnose microscopically, but typical signs are cell vacuolization, hyperchromatic nuclei, and protein denaturation [10]. The extent of tissue damage depends firstly on the duration of the laser’s application on that tissue and, secondly, on the laser power. Moreover, laser application time increases if multiple metastases within a limited area (i.e., a lobe) require resection. The heated tissue cools down slowly once the laser intervention is over, at least if the lung is not mechanically cooled down intraoperatively. It was therefore hypothesized that the lung tissue cools down by releasing heat into the environment and via the pulmonary vessels. To date, there is no data evaluating the cooling time and the cooling mechanism after laser-directed lung resection. In the presented study, we attempted to answer these questions using a specially designed experimental pig lobe model.
Materials and methods
Pig lung model and experimental setup
Paracardiac pig lung lobes (n = 12) of a mean weight of 46 ± 5 g were dissected from freshly slaughtered adult pigs at the slaughterhouse. The pulmonary arteries supplying the lobes were identified and directly cannulated with a 12-G catheter. Via this cannula, normothermic heparinized saline (5,000 IE) was immediately injected to flush out any blood clots from the lobe vessels. This perfusion was stopped when the perfusate exiting the lobe vein was clear. After removal, the lobes were wrapped in a moist compress, packed into a bag with a temperature at 37 °C, and immediately transported to the laboratory. Transportation time to the laboratory was only 10 min. Ambient laboratory temperature was constant at 25 °C and regulated by a thermostat. All lobes were fixed vertically and perfused with a saline solution at 37 °C until the parenchyma was homogenously perfused and showed uniform normothermic temperature distribution. The impermeability of the cannula and the lung parenchyma as well as the perfusate outflow from the lobe vein was checked by inspection. We perfused the lobes with a constant hydrostatic perfusion pressure of 25 cm H2O (18.5 mmHg). In former unpublished studies, we measured a flow rate of 12.21 ± 3.79 ml/min. The lobes were monitored with an infrared camera (Type IC 120LV; Trotec, Heinsberg, Germany) for homogeneous, normotensive perfusion for 5 min before laser application. To ensure that the same parenchymal amount was consistently resected in a reproducible mode, a piece of the lung parenchyma (area 2.5 cm2) was clamped with a Satinsky clamp for marking the resection line, and after which, the clamp was removed. Resection was done along the marked parenchyma line with the Nd:YAG laser LIMAX® 120 (Gebrüder Martin GmbH & Co. KG, Tuttlingen, Germany) with a laser output of 100 W. The Nd:YAG LIMAX® 120 laser system is a noncontact diode-pumped laser (optimal focus distance to the tissue surface is 30 mm) with a wavelength of 1,318 nm. Temperature was continuously monitored using the infrared camera and a freeze-frame recorded at 10-s intervals from which the temperature values for further analysis were obtained. The infrared camera (Type IC 120LV; Trotec, Heinsberg, Germany) provides a 384 × 288 infrared sensor with 110,592 independent temperature measure points. It is able to measure the temperature at a range between −20 and +1,500 °C with high precision (thermic precision of 0.08 °C). The camera was fixed on a tripod at a constant distance from the resection area. The experiment was stopped when the lung surface had reached a temperature of 37 °C measured by the thermal imaging camera.
Treatment groups
We examined two groups, each containing six lobes allocated at random. After reaching normothermia, perfusion of the lobes in group 1 was stopped, and resection was performed without any additional perfusion. In group 2, lobes were perfused as indicated, and perfusion was continued during laser resection and afterwards.
Statistical analysis
We did explorative experiments in the planning of this study. With these data and the high effect size, we ran a power analysis (program G*Power 3, Institute of Experimental Psychology, Heinrich Heine University, Düsseldorf, Germany) using a nonparametric calculation. With this procedure, we calculate our sample size n = 6 for each group. Values are represented as mean ± standard deviation unless otherwise indicated. Group comparisons were done using the Mann–Whitney test, and p < 0.05 was considered statistically significant. To compare the temperature decay curves mathematically, we conducted a nonlinear fit analysis using the “one-phase exponential decay” algorithm provided by Prism 5 software (GraphPad Prism 5, La Jolla, CA, USA).
Results
Using the paracardial pig lung lobes and marking the resection area using a Satinsky clamp, nonanatomical resections of a standard area of approximately 2.5 cm2 could be performed reproducibly, and the resection could be completed within a median time of 15 ± 2 s, which did not differ significantly between the two groups. There were no significant differences of laser exposure time between the two groups. Immediately after completing resection, temperature distribution at the resection surface showed a concentric pattern, with highest temperatures at the surface center and dropping slightly towards the margins (Fig. 1). At a distance of approximately 1.5 cm from the center, the temperature levels reached physiologic levels. To ensure reproducible results and avoid bias, we used the peak temperature within the surface area for all further analyses.
Following resection, temperature decay was recorded every 10 s using the infrared camera until the resection surface area in the remaining lung tissue reached a temperature of 37 °C. Mean temperature at the center of the resected lung surface immediately after completion of the laser resection was 84.49 ± 10.95 °C in group 1 and 76.75 ± 5.33 °C in group 2 (p = 0.29; Fig. 2, Table 1). A postresection temperature of 37 °C in the remaining lung tissue surface was reached after 182.95 ± 53.76 s in group 1 and 121.70 ± 16.02 s in group 2 (p = 0.01; Figs. 3 and 4). As a surrogate marker for tissue damage, we calculated the mean time, and the tissue at the resection surface was exposed to temperatures of >42 °C. Temperatures above 42 °C were sustained for 97.14 ± 26.90 s in group 1 and for 65.00 ± 13.78 s in group 2 (p = 0.02; Fig. 4).
Temperature decay in group 1 (no perfusion) and group 2 (perfusion). The initial temperature immediately after completing the resection differed in the two groups, showing a lower temperature in the perfused lobes. During the cooling process, the temperatures followed a similar course. The dotted line represents a temperature of 37 °C
a Quantification of the time needed for the tissue at the resection margin to reach normothermia/37 °C. b The duration the tissue was exposed to temperatures of >42 °C. Group 2 cooled down to 37 °C significantly sooner and was exposed to temperatures of >42 °C which are significantly shorter than those in group 1. *p < 0.05
Over the whole course of the cooling, both groups’ mean temperatures dropped following a nonlinear curve, which we quantified via a nonlinear curve fit. Based on the data, formulas describing the curves for each of the two groups were calculated as follows: group 1, y = 43.00 ∗ e − 0.028x + 37.98, and group 2, y = 40.13 ∗ 13e − 0.026x + 35.45 (x, time in seconds; y, measured temperature in degree Celsius) and were plotted (Fig. 5). Difference between the curves is mainly due to a shift to higher initial temperatures in group 1, indicating that the perfusion keeps the resected area cooler when compared to nonperfused lobes. However, temperature decay in both groups shows a parallel course, indicating that at this stage, tissue emission is predominant and perfusion does not accelerate heat dissipation.
Looking at the histologic examination (HE staining) of the resected lung areas using laser, there were no morphologic differences between the two groups (Fig. 6). The histologic slides showed a homogeneous coagulation zone with a similar mean maximum depth of 1.9 ± 0.3 mm (range, 1.7–2.1 mm) in both groups. The lung tissue below this coagulation zone had no histologic signs of thermal damage.
Discussion
The present study clearly shows that the lung tissue surrounding the laser resection line heats up considerably (up to 85 °C) during each laser application. In the presented experimental model, we could demonstrate for the first time that the laser-induced initial tissue temperature at the resection margin was not significantly different between the two groups (76.75 vs. 84.49 °C, p = 0.29). There was an effect of the lung perfusion regarding the normalization of the tissue temperature after laser resection. To reach a baseline temperature of 37 °C, it took 121.7 s with and 182.9 s without perfusion (p = 0.01). The time course of cooling was nearly similar in both groups, and after 60 s, there was a significant drop of the measured temperature in the perfusion group (Table 1). Looking at the surrogate marker for tissue damage, i.e., the time the tissue is exposed to temperatures of >42 °C, there was also a significant difference between the two groups (p = 0.02). Based on these data, we conclude that tissue perfusion played some role in heat dissipation, mainly by keeping lobes cooler during laser procedure, but the predominant mechanism of heat dissipation after laser resection was heat emission to the environment, indicated by the lack of an accelerated temperature decline in the perfused lobes. About only 28 % of the whole heat dissipation was caused in our model by perfusion. This may be explained by the fact that the lasered area was located at the lung surface and thus not supplied by large vessels and high blood flow. Moreover, small vessels supplying the resected area are most likely coagulated by the laser beam resulting in a negligible perfusion on the resection surface, as evidenced by a lack of fluid loss through the resection surface during or after laser procedure. Most of the dissipated heat must originate from more centrally located lung areas. There were no further studies in the literature on this topic. Our study was performed in an ex vivo model and without blood perfusion. Therefore, in an in vivo model, the role of blood perfusion for the heat dissipation could become more important, so further studies are necessary to investigate this issue, especially because the property of blood is different compared with that of saline with respect to inducing capillary or small vessel blockage. The presented data are important to consider when several laser resections are done within a small area, i.e., within a single lobe, an intervention associated with the risk of continuous heat accumulation from the laser and continuous thermic damage to the remaining healthy lung parenchyma. A potential consequence would be to take about 3–5-min breaks in between laser resections to give the lung parenchyma time to cool off. Also, we suggest that there is a need for further research, finding an effective cooling method for the lung tissue. For this, different approaches could be employed. Precooling of the lung parenchyma prior to laser resection could be an interesting method. Al et al. [11] showed that the thermal tissue damage on oral mucosa during CO2 laser surgery may be reduced by precooling. They also showed that precooling was associated with a lesser degree of immediate mast cell degranulation as a surrogate marker for tissue damage.
Since cooling the lung tissue by perfusion of the pulmonary artery is a substantial effort and rather ineffective, topical cooling during or immediately after the laser application may also be a successful cooling strategy. Beer et al. [12] showed in an in vitro study on pork liver using a 980-nm diode laser in micropulsed mode that water/air cooling during laser application has an effect on collateral tissue damage. Koo et al. [13] examined whether a novel vortex cooling device that generates cooled air at low flow rates (3 l/min) provides a cooling benefit during simulated laryngeal laser surgery using a continuous-wave thulium laser. He showed by histologically examinations that this device was able to reduce the thermal tissue damage. Maybe this could be also a cooling strategy for the lung tissue; unfortunately, no data exist yet about this issue. Cooling after laser resection by submerging the lung in cold water for a definite amount of time could be an easy-to-handle method; however, as we mentioned earlier, there were also no data available about this issue. With this, we planned further studies investigating the optimal cooling strategy for laser application on lung tissue.
Conclusion
Heat dissipation after nonanatomical resection using a diode-pumped Nd:YAG laser is predominantly caused by emission of the heat to the environment. With respect to cooling strategies, a topical cooling method will most likely yield the greatest success.
References
Osei-Agyemang T, Ploenes T, Passlick B (2012) Pulmonary metastasectomy: indication and technique. Zentralbl Chir 137:234–241
Friedel G, Pastorino U, Buyse M (1999) Resection of lung metastases: long-term results and prognostic analysis based on 5206 cases—the International Registry of Lung Metastases. Zentralbl Chir 124:96–103
Harrison-Phipps K, Cassivi S, Nichols F, Allen M, Pairolero P, Deschamps C (2005) Conventional resection of pulmonary metastases. MMCTS 001818:1–7
Venuta F, Rolla A, Anile M, Martucci N, Bis B, Rocco G (2010) Techniques used in lung metastasectomy. J Thorac Oncol 5:S145–S150
Rolle A, Pereszlenyi A (2004) Laser resection of lung metastases. MMCTS 000570:1–7
KLS Martin Group (2013) Product informations about Nd:YAG Laser LIMAX® 120 system. www.klsmartin.com. Accessed 20 Apr 2013
Sonntag PD, Hinshaw JL, Lubner MG, Brace CL, Lee FT (2011) Thermal ablation of lung tumours. Surg Oncol Clin N Am 20:1–20
Zervas NT, Kuwayama A (1972) Pathological characteristics of experimental thermal lesions. J Neurosurg 37:418–422
Nikfarjan M, Muralidharan V, Christophi C (2005) Mechanisms of focal heat destruction of liver tumours. J Surg Res 127:208–223
Ross E, Uebelhoer N (2011) Laser-tissue interactions. In: Nouri K (ed) Lasers in dermatology and medicine. Springer, London, pp 1–21
Al P, Browne RM, Frame JW, Matthews JB (1993) Assessment of thermal damage in precooled CO2 laser wounds using biological markers. Br J Oral Maxillofac Surg 31:239–243
Beer F, Körpert W, Buchmair AG, Passow H, Meinl A, Heimel P, Moritz A (2013) The influence of water/air cooling on collateral tissue damage using a diode laser with an innovative pulse design—an in vitro study. Lasers Med Sci 28:965–971
Koo HJ, Burns JA, Kobler JB, Heaton JT, Zeitels SM (2012) Novel devices for tissue cooling during endoscopic laryngeal laser surgery: thermal damage study in an ex vivo calf model. Ann Oto Rhinol Laryngol 121:485–489
Acknowledgments
We thank PD Dr. Helmut Sitter, Biostatistician, University Hospital Giessen and Marburg GmbH, for his help and support in the statistical analyses of this study.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kirschbaum, A., Ocker, M., Bartsch, D.K. et al. Heat dissipation after nonanatomical lung resection using a laser is mainly due to emission to the environment: an experimental ex vivo study. Lasers Med Sci 29, 1037–1042 (2014). https://doi.org/10.1007/s10103-013-1460-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1460-9