Abstract
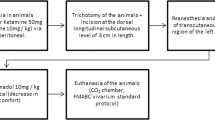
The aim of the present study was to compare the effectiveness of four different laser wavelengths (660, 810, 980, and 1,064 nm) used for low-level laser therapy (LLLT) on the healing of mucositis in an animal model of wound healing by investigating the expression of platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and blood-derived fibroblast growth factor (bFGF). Thirty-five male Wistar albino rats with a weight of 250–300 g body mass and 5 months old were used in the study. All animals were intraperitoneally injected with 100 mg/kg of 5-fluorouracil (5-FU) on the first day and 65 mg/kg of 5-FU on the third day. The tip of an 18-gauge needle was used in order to develop a superficial scratching on the left cheek pouch mucosa by dragging twice in a linear movement on third and fifth days. After ulcerative mucositis were clinically detected on the animals' left cheek pouch mucosa, the laser therapy was started. Four different laser wavelengths (660 nm, HELBO, Bredent; 810 nm, Fotona XD, Fotona; 980 nm, ARC Fox; and 1,064 nm, Fidelis Plus 3, Fotona) used for LLLT at ED 8 J/cm2 daily from the first to the fourth days. Oval excisional biopsy was taken from the site of the wound, and the expression of PDGF, TGF-β, and bFGF was evaluated. The obtained data were analyzed by one2-way ANOVA, and then Tukey HSD tests were used for pairwise comparisons among groups (α = 0.05). The one-way ANOVA test indicated that expression values of the growth factors, PDGF and bFGF, were significantly affected by irradiation of different wavelengths of lasers (p < 0.001). However, expression value of the TGF-β was not affected by irradiation of different wavelengths of lasers (p > 0.05). The highest PDGF expression was detected in neodymium-doped yttrium aluminum garnet (Nd:YAG) laser group (p < 0.05), and there were no statistically significant differences among the other groups (p > 0.05). The highest bFGF expression was detected in 980-nm diode and Nd:YAG laser groups (p < 0.05), and there were no statistically significant differences among the other groups (p > 0.05). These findings suggest that low-level Nd:YAG and 980-nm diode laser therapy accelerate the wound healing process by changing the expression of PDGF and bFGF genes responsible for the stimulation of the cell proliferation and fibroblast growth.

Similar content being viewed by others
References
Kwong KK (2004) Prevention and treatment of oropharyngeal mucositis following cancer therapy: are there new approaches? Cancer Nurs 27:183–205
Eilers J (2004) Nursing interventions and supportive care for the prevention and treatment of oral mucositis associated with cancer treatment. Oncol Nurs Forum 31(4 Suppl):13–23
Fulton JS, Middleton GJ, McPhail JT (2002) Management of oral complications. Semin Oncol Nurs 18:28–35
Kirsner R (2003) Wound healing. In: Bolognia J, Jorizzo J, Rapini R (eds) Dermatology. Mosby, Bolognia, pp 2207–2218
Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M (2008) Effects of low-level He–Ne laser irradiation on the gene expression of IL-1beta, TNF-alpha, IFN-gamma, TGF-beta, bFGF, and PDGF in rat's gingiva. Lasers Med Sci 23:331–335
Abbas AK, Lichtman AH, Pober JS (1999) Cellular and molecular immunology, chapter 3. Saunders, Philadelphia
Cameron MH, Perez D, Otaho-Lata S (1999) Electromagnetic radiation. In: Cameron MH (ed) Physical agents in rehabilitation: from research to practice. Saunders, Philadelphia, pp 303–344
Webb C, Dyson M, Lewis WHP (1998) Stimulatory effect of 660 nm level laser energy on hypertrophic scar derived fibroblasts: possible mechanisms for increase in cell counts. Lasers Surg Med 22:294–301
Agaiby AD, Ghali LR, Wilson R, Dyson M (2000) Laser modulation of angiogenic factor production by T Lymphocytes. Lasers Surg Med 26:357–363
Leung MC, Lo SC, Siu FK, So KF (2002) Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and upregulates the expression of transforming growth factor-Beta. Lasers Surg Med 31:283–288
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low-level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Carnevalli CM, Soares CP, Zângaro RA, Pinheiro AL, Silva NS (2003) Laser light prevents apoptosis in Cho K-1 cell line. J Clin Laser Med Surg 21:193–196
Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66:866–871
Stadler I, Evans R, Narayan V, Buehner N, Naim JO, Lanzafame RJ (2001) 830 nm irradiation increases wound tensile strength in a diabetic murine model. Lasers Surg Med 28:220–226
Karu T (2010) Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg 28:159–160
Anneroth G, Hall G, Ryden H, Zetterqvist L (1988) The effect of low-energy infra-red laser radiation on wound healing in rats. Br J Oral Maxillofac Surg 26:12–17
Cambier DC, Vanderstraeten GG, Mussen MJ, van der Spank JT (1996) Low-power laser and healing of burns: a preliminary assay. Plast Reconstr Surg 97:555–558
Walker MD, Rumpf S, Baxter GD, Hirst DG, Lowe AS (2000) Effect of low-intensity laser irradiation (660 nm) on a radiation-impaired wound- healing model in murine skin. Lasers Surg Med 26:41–47
Schlager A, Kronberger P, Petschke F, Ulmer H (2000) Low-power laser light in the healing of burns: a comparison between two different wavelengths (635 nm and 690 nm) and a placebo group. Lasers Surg Med 27:39–42
Al-Watban FA, Delgado GD (2005) Burn healing with a diode laser: 670 nm at different doses as compared to a placebo group. Photomed Laser Surg 23:245–250
Al-Watban FA (2009) Laser therapy converts diabetic wound healing to normal healing. Photomed Laser Surg 27:127–135
Jahangiri Noudeh Y, Shabani M, Vatankhah N, Hashemian SJ, Akbari K (2010) A combination of 670 nm and 810 nm diode lasers for wound healing acceleration in diabetic rats. Photomed Laser Surg 28:621–627
Cury V, Bossini PS, Fangel R, Crusca Jde S, Renno AC, Parizotto NA (2009) The effects of 660 nm and 780 nm laser irradiation on viability of random skin flap in rats. Photomed Laser Surg 27:721–724
de Oliveira Guirro EC, de Lima Montebelo MI, de Almeida BB, da Costa Betito Torres MA, Polacow ML (2010) Effect of laser (670 nm) on healing of wounds covered with occlusive dressing: a histologic and biomechanical analysis. Photomed Laser Surg 28:629–634
Bensadoun RJ, Nair RG (2012) Efficacy of low-level laser therapy (LLLT) in oral mucositis: what have we learned from randomized studies and meta-analyses? Photomed Laser Surg 30:191–192
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Genot MT, Klastersky J (2005) Low-level laser for prevention and therapy of oral mucositis induced by chemotherapy or radiotherapy. Curr Opin Oncol 17:236–240
França CM, França CM, Núñez SC, Prates RA, Noborikawa E, Faria MR, Ribeiro MS (2009) Low-intensity red laser on the prevention and treatment of induced-oral mucositis in hamsters. J Photochem Photobiol B 94:25–31
Takamiya M, Saigusa K, Nakayashiki N, Aoki Y (2003) Studies on mRNA expression of basic fibroblast growth factor in wound healing for wound age determination. Int J Legal Med 117:46–50
Ihn H (2002) Pathogenesis of fibrosis: role of TGF-b and CTGF. Curr Opin Rheumatol 14:681–685
Heldin CV, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316
Hallén S, Brzezinski P (1994) Light-induced structural changes in cytochrome c oxidase: implication for the mechanism of electron and proton gating. Biochim Biophys Acta 1184:207–218
Kato M, Shinzawa K, Yoshikawa S (1981) Cytochrome oxidase is a possible photoacceptor in mitochondria. Photochem Photobiophys 2:263–269
Pereira AN, Eduardo Cde P, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Almeida-Lopes L, Rigau J, Zangaro RA et al (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Hawkins D, Abrahamse H (2007) Influence of broad-spectrum and infrared light in combination with laser irradiation on the proliferation of wounded skin fibroblasts. Photomed Laser Surg 25:159–169
Al-Watban FA, Zhang XYA (1996) Comparison of the effects of laser therapy on wound healing using different wavelengths. J Laser Ther 8:127–135. https://www.jstage.jst.go.jp/article/islsm/8/2/8_2_127/_article.
Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD (2004) The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg 22:323–329
Al-Watban FA (2004) The comparison of effects between pulsed and CW lasers on wound healing. J Clin Laser Med Surg 22:15–18
Karu T (2002) Low-power laser effects. In: Waynant RW (ed) Lasers in medicine. CRC, Boca Raton, pp 171–210
Houreld N, Abrahamse H (2007) In vitro exposure of wounded diabetic fibroblast cells to a Helium–Neon laser at 5 and 16 J/cm2. Photomed Laser Surg 25:78–84
Acknowledgments
The author wishes to express their sincere appreciation to ARC Fox and HELBO companies for supplying the laser devices for this study and to Jan Tunér and René-Jean Bensadoun for sharing their knowledge and experiences about LLLT.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is based on the Master Thesis in “Lasers in Dentistry” in RWTH Aachen University.
Rights and permissions
About this article
Cite this article
Usumez, A., Cengiz, B., Oztuzcu, S. et al. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci 29, 1807–1813 (2014). https://doi.org/10.1007/s10103-013-1336-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1336-z