Abstract
The aim of this experimental study, process simulation and economic analysis is to assess the applicability of pyrolysis technology for processing end-of-life tyres and to evaluate the economic viability of a 60 ton/day EOLT processing facility: a case-specific study within Australia. The experimental work and characterization of feedstock and products were carried out in-house. Capital costs for major equipment were collected from suppliers. The running cost of the processing facility is calculated on the basis of the current labour and utility costs. An economic model is developed based on the information generated from the experimental program and those obtained from suppliers. From the analysis, it is evident that the pyrolysis process for processing EOLT promises a significant upside in economic terms. A conservative conclusion of 20% light oil, ~ 65% furnace oil and 7% carbon black, generated as pyrolysis products, depicts a cash-flow positivity for a 60 tonne per day (TPD) plant that can be run using the generated fuel gas for under 4 years. This is in addition to the benefit of the zero landfill requirement. Apart from the base calculations, the sensitivity of six different scenarios is analysed by mainly changing the land cost and bank investment. Depending on the scenario, the calculated internal rate of return varies between 15 and 35%. While Australia generates significant quantities of EOLT, the techno-economic results confirm that pyrolysis technology for processing EOLT is a viable solution in Australia. However, a dedicated supply chain needs to exist to make pyrolysis plants an attractive investment at defined locations.
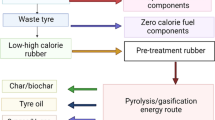
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advancements in technology over the years and certainly the twenty-first century have now placed our generation in one of the most blossoming eras of our time. However, if one was to flip the coin and study the dark side of development, it is quite evident and alarming to find ourselves in a precarious situation. A question that deeply bothers researchers and industrialists for a considerable amount of time is how we address the demands of an ever-growing population with their needs perpetually growing and yet find novel and sustainable ways to reduce the waste that is generated.
The race to find alternative sources of fuel, as an effort to reduce the dependency on imported fossil fuels, is on the charge. A question that inspired many different studies around the world is: How can one add value to an end of life product and is there a possibility to create a feasible solution that can be scaled and commercialized?. Drawing inspiration from the above question inspired a series of studies in our laboratory to understand the processing of end-of-life tyres (EOLT) into a source of high-quality fuel and other products comparable to what is being used in the market today and other products. The tyre and tube wastes are composed of rubber, which is not easily biodegradable. An EOLT is defined as a product that can no longer be used in vehicles and those that have served their purpose in all its entirety, often even after re-treading. EOLTs primarily consist of carbon-rich compounds (up to 85 wt%) composed of “natural rubber (NR), synthetic rubber (SR, which includes styrene-butadiene rubber 'SBR' and butyl rubber 'BR'), carbon black (CB), steel, and some organic and inorganic fillers” as described in Martinez (2021). Industrially, tyres are classified on the basis of their size, which categorizes them as either truck (TTs) or passenger car (PCTs) as described in Martinez (2021). Truck tyres have a greater composition of NR due to their unique inherent properties that provide higher mechanical resistance and thermal stability, while several different kinds of CB are utilized to provide the required mechanical strength for the tyres to increase their durability when used on different kinds of roads as discussed in Martinez (2021). Additionally, the steel cords bolster the rigidity and resistance, mainly in radial tyres. The different variations of these components that make up a tyre determine the elemental composition of carbon and sulphur (the main component of the vulcanization process, which is added to initiate the cross chains in the rubber structure as described in Martinez (2021), which further determines the type of tyre it finally becomes and subsequently the inherent challenges in recovery and reuse.
Over the previous five years to 2021, Australia generated an average of 450,000 tonnes of EOLT, or approximately 56 million units annually as reported by the Tyre Stewardship Australia (2021). Approximately 69% of this EOLT was recovered for reuse and processed into tyre-derived solid products (TDP: the segregation of EOLT into a range of products, which includes shredded, baled, steel, fabric and rubber), and the remainder was exported, landfilled or stockpiled, similar to many of the countries around the globe. Furthermore, a series of data collected and analysed during an industry survey sheds light on the recycling fates of crumb rubber, which accounts for nearly half of all domestic recycling (approximately 22,000 tonnes), wherein the reuse of these crumbs in steel accounts for an additional 16,000 tonnes and repurposing for civil engineering jobs account for approximately 5000 tonnes per year Genever et al. (2017). As a result, the lack of management of EOLT due to stockpiling and dumping poses a serious risk of causing tyre fires, raising situations where harmful emissions of toxic gasses are released into the environment while also polluting the groundwater from the contaminated runoff as the fire is extinguished.
Following a review paper by Martinez (2021), we observe that the above-addressed issue is a concern of matter globally and not just within Australia. The author highlights the generation of EOLTs in a few Latin American countries, such as Argentina (150,000 t/year, approximate population of 44 million), Brazil (588,000 t/yr, approximate population of 214 million) and Mexico (468,000 t/yr; an approximate population of 130 million), to name a few, to be some of the highest EOLT-producing nations in that region Martinez (2021).
When stripped of metal, tyre is a carbon and hydrogen-containing material. Therefore, the inherent energy in end-of-life tyres can be harnessed through their processing by various means, in particular thermochemical or thermo-catalytic means. An experimental and economic analysis study, which are uncommon, was therefore undertaken. This paper presents the key results of this study. The exact objectives are stated following the literature review in the next section.
Literature review
In searching the significance of EOLT processing within Australia, we notice New South Wales (NSW) as the largest generator of EOLT with approximately 127,000 tonnes (~ 16 million EPUs; Equivalent Passenger Unit: 1 EPU = 8 kg), followed by Victoria and Queensland generating approximately 107,500 tonnes (~ 13.4 million EPUs) and 96,300 tonnes (~ 12 million EPUs) in 2015–2016, respectively, as reported in Genever et al. (2017). These figures should not come by as a surprise, given that the generation of EOLT is closely related to the population distribution across these states. While assuming that the per capita generation of EOLT remains the same, it can be projected that, by considering population growth and tyre consumption, the EOLT numbers will cross 61.5 million EPU by the end of 2025. After NSW, the state of Victoria is the largest producer of EOLT at approximately 13.4 million EPUs, as reported in Supplementary Information Table S.1.
Furthermore, reports have highlighted the significant increase in the exports of used and in-use EOLT and TDP from Australia, which surged by approximately 170% in 2015 when compared to previous years, with the highest exporter of used being the state of Victoria as reported in Genever et al. (2017). With that being said, this provides a window of opportunity to exploit a market with high potential for TDPs in Australia, which is estimated to increase their current recovery rate by more than 50% by 2025–26 as reported in Genever et al. (2017). The current applications of TDP are mainly in the construction of roads, railways and other civil engineering—related fields, wherein tyre rubber crumbs are mixed in asphalt and used in spray seal production. However, there are several emerging markets that were previously overlooked for the reuse of EOLT, such as crumb rubber explosives, rubberized concrete and tyre pyrolysis, which are underway and expected to flourish in the near future, with Supplementary Information Table S.2 providing a summary of the various forms of TDP and their applications.
In addition to these applications as stipulated in Table S.2, another possible procedure to recover energy from tyre scraps is through a well-known process called pyrolysis, a process known to us since the bronze age, which was initially implemented in the synthesis of vegetable charcoal. Following Czajczyńska et al. (2017), pyrolysis (also known as thermolysis) is a thermal conversion technique that includes dehydration, cracking, isomerization, dehydrogenation, aromatization and condensation of the feedstock in an inert atmosphere (oxygen-free), which results in a no-oxidation process. The mechanism that overarches this particular process is of significant importance during reactor design and desired product profiles Kumaravel et al. (2016). Following their research, there are two stages in the pyrolysis process:
Stage 1: Primary pyrolysis: Vapour and volatile products composed of different hydrocarbons are produced in this stage.
Stage 2: Secondary cracking: converting the primary products into compounds that may have a higher market value through processes such as aromatization.
The thermal decomposition of polymers, such as NR, can be attributed to one of the four mechanisms that have been identified in Kumaravel et al. (2016). These are random chain scission; end chain scission; chain stripping and cross-linking. In a recent review published by Arabiourrutia et al. (2020), the author summarized the different possible reactors that have been used in previous studies for the EOLT pyrolysis process, including fixed bed, fluidized bed, spouted bed, rotary kiln, auger and twin auger Martínez et al. (2020), and microwave reactors. It was also reported that most of the existing technologies have their own inherent challenges, with many of them being either batch or semi-batch operations. However, in our recent investigations, we have studied the possibility of using a continuous stirred tank operation, with the relative ease of operation process, scalability and high-grade quality of the pyrolysis products formed, as reported in later sections of this study.
In addition to these findings, it has also been reported in Kumaravel et al. (2016) and Wey et al. (1995) that the pyrolysis process is governed by parameters such as temperature, pressure, type of gaseous environment and the retention time of the volatile compounds in the reaction zone. In a review published by Martínez et al. (2013), the authors present an argument that favours pyrolysis over other thermochemical processes due to its minimal impact on the environment and in addition to the recovery of both liquid and solid materials from the process. This is corroborated by other researchers as in Galvagno et al. (2002) and Aylón et al. (2005). Pyrolysis draws its popularity from the concept that waste valorisation is recognized as an efficient rather than a destructive process in which the products are easily manageable; therefore, over the years, a few pyrolysis plants have been established worldwide, particularly in Germany, China, the Netherlands and Canada, as shown in Supplementary Information Table S.3, using batch and fixed bed continuous reactors at capacities between 4 and 200 tonnes/day.
Following Table S.3, currently, there are only a few tyre pyrolysis pilot-scale plants operating in Australia.Footnote 1 The major reasons behind this shortage may be credited to the limited fundamental knowledge associated with feedstock properties, process requirements and adaptability of the pyrolysis process for solid feedstocks. Recently, an economic study conducted by Ghodrat et al. (2019), encompassing the utility of waste plastic for energy recovery using the pyrolysis technique from an Australian perspective, was published in Ghodrat et al. (2019). The outcomes of this study affirmed that the pyrolysis treatment of waste plastics is a viable option in Australia from a technical as well as economic point of view. However, to the best of our knowledge, until now, there are no studies that combine actual experimental data to process EOLT using pyrolysis technology with an economic analysis from an Australian context.
This research addresses the gap in the literature in Australian context. We focus on the pyrolysis of EOLT for energy recovery and its related economic analysis in case of Australia. Two major research contributions are:
-
First, to assess the applicability of pyrolysis technology for processing EOLT.
-
Second, to evaluate the economic viability of using pyrolysis for processing EOLT in the Australian context.
To achieve these objectives, it is imperative to determine the yield and composition of the pyrolysis products procured from EOLT. For this very reason, and to generate scientific and engineering information that can further aid in developing a process model for subsequent economic analysis, experimental work was carried out in three stages:
Stage 1: Thermogravimetric analysis (TGA) to determine the breakdown temperature of the tyre granules.
Stage 2: A large bench-scale continuously stirred reactor with online gas and oil sampling operated in a steady state.
Stage 3: Different analytical methods and instruments were applied to generate necessary information regarding the pyrolysis products for process development in terms of heat and mass balance.
To convey a better understanding of the entire process, this study aims to analyse a specific case of an EOLT processing facility in Victoria, Australia, which is modelled to process 60 tonnes/day (TPD) in 2021 based on our own experimental data and cost data collected from a vendor building such plants. Therefore, the subsequent work focuses on presenting a case study-based economic assessment while highlighting the important variables influencing the feasibility of the proposed EOLT processing plant.
Methodology
This section of the study describes the stepwise methods adopted in the study.
Step 1: Feedstock characterization
The samples processed for this particular study were metal-separated shredded provided by Tyre Stewardship Australia. The size of the shredded granules was 6–10 mm. Proximate and ultimate analyses of the samples were carried out since the compositional differences between the different kinds of tyre feedstocks will result in differences in product distribution during pyrolysis as reported in Arabiourrutia et al. (2020).
The proximate analysis (on a dry basis) of the sample was accomplished using a TGA.Footnote 2Approximately 10 ± 1 mg of the sample was analysed in the TGA, where the temperatures were raised up to 600 °C at a heating rate of 5 °C/min. Additionally, the ultimate analysis (dry basis) was completed using a FLASH 2000 Ultimate analyser (Thermo Scientific, Milan, Italy).
The ash composition of the tyre sample was measured using X-ray fluorescence spectroscopy (XRF) (XRF-EDX-720: Shimadzu Corp, Kyoto, Japan) on a carbon-free basis with a voltage of 15–50 kV in an air atmosphere. The fluorescence spectra procured from XRF were further analysed to identify the elements present within the carbon-free ash, which was prepared by combusting the sample at 60 °C.
Moreover, to study the surface composition of the residual char obtained from the pyrolysis reactor, scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM–EDX) was implemented to study the morphologies and for the semiquantitative identification of the minerals present in the residues from the pyrolysis reactor.
Step 2: Continuous stirred tank pyrolysis
A total of six tests were conducted in a large bench-scale continuously fed pyrolyser. In each test, approximately 0.5 kg of the feedstock was preloaded into the reactor. The reactor temperature was raised from room temperature up to 450 °C, taken as the average of thermocouples T1, T2 and T3 in Fig. 1. The temperature range for the experiment was inferred from thermogravimetric analysis (TGA) results of the scrap tyre presented in Sect. 4.2. The reactor was coupled with a condenser to quench the condensable crude liquid produced during the process. A schematic diagram of the reactor with the auxiliaries is presented in Fig. 1.
Schematic diagram and picture of the bench-scale pyrolyser (adapted with permission from Elsevier) (Auxilio et al. 2017)
After the initiation of the pyrolysis process at the set temperature, the evolved gas was cooled in the condenser for liquid collection, and the light gas exits the process line through a vent channel. Continuous feeding of the feedstock was then initiated at 1 kg/hr. Gas bags were used to collect the sample gas for gas chromatographic analysis in a Varian 490-GC Micro-GC equipped with Molsieve-5A and PoraPlot Q columns and a thermal conductivity detector, where nitrogen was used as an internal standard for chromatographic analysis. The char remains inside the reactor, which is collected after the reactor was cooled to room temperature. The reactor is coupled with its own catalytic distillation column; however, the catalytic distillation column was not used to favour the conditions of a conventional distillation column.
The condensed liquid was stored in vented-cap glass bottles and subjected to further distillation in a 300-mm-long Vigreux distillation column coupled with heating mantle, which was built to facilitate the distillation process of the crude liquid. The composition of the oil was analysed in a Perkin Elmer Clarus 600 gas chromatograph coupled with a Clarus 600S mass spectrometer (GC–MS). The crude oil was distilled at 230 °C, approximately 30 °C below the boiling point of light oil. There is, however, a possibility for using different distillation temperatures to maximize the yield of the light oil fraction.
Results and discussion
Following the above instrumental analysis, the raw data were collected and interpreted and compared with the current studies in the literature. The subsections hereafter collate the data and present our analysis of the experimental work carried out in a continuous stirred tank reactor, highlighting the quality of the pyrolysis products and operating parameters.
Analysis of shredded EOLT samples
The overall analysis of the tyre sample is provided in Supplementary Information Table S.4, which includes the proximate, ultimate (elemental) and ash analysis. Inferring from the proximate analysis, it can be understood that the high content of volatiles (63.30 wt%) provides the impetus to highlight the potential of the pyrolysis process for their valorisation, while the fixed carbon content (30.50 wt%) can be directly associated with the carbon black that has been included in their manufacturing. Our findings support Arabiourrutia’s (2020) research. The ash content indicates the concentration of the metallic additives that were present after the removal of the steel.
The high contents of carbon (83.10%) and hydrogen (7.38 wt%) promote the production of hydrocarbon fuels and hydrogen during the pyrolysis process, whereas the sulphur content points to the likely presence of sulphur compounds in the pyrolysis products.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed to identify the range of breakdown temperatures, which was utilized to set the pyrolysis reactor temperature conditions. The TGA curves provide signals of weight loss as a function of temperature. The first derivative of the thermogravimetric curve called the differential thermogravimetry (DTG) helps in identifying the breakdown temperatures of the feedstock sample. The results, shown in Supplementary Information Fig. S.5, are utilized to understand the temperature range in which most of the polymers/compounds in the shredded tyre samples will breakdown as a function of temperature. It can be inferred from the TGA and DTG graphs that the sample begins to decompose at approximately 200 °C and completes at approximately 550 °C when subjected to a heating rate of 5 °C /min.
The highest degree of decomposition is observed at approximately 350 °C, with 35 wt% of the total sample weight remaining as a solid residual. The results reported below are consistent with similar published TGA results for tyre and tyre components Wlliams and Besler (1995).
Dividing the TGA curve into three main weight loss stages within the 200–600 °C temperature range, as published in the existing literature as in Seidelt et al. (2006) and Choi et al. (2017), it can be concluded that the thermal degradation at 200–350 °C corresponds to the devolatilization of additives that are utilized in the initial manufacturing process, such as vulcanization agents, plasticizers and oils. The temperature range of 350–450 °C corresponds to the mass loss region of NR and SBR, which are the main manufacturing components of tyres, whereas the region between 450 and 500 °C is related to the decomposition of SBR Choi et al. (2017).
Assessment of the pyrolysis products
The pyrolysis products of EOLT are mainly composed of three components: combustible gases (gas phase products), condensable crude liquid (liquid phase product) and solid char (solid phase product). The liquid phase product was further subjected to a subsequent distillation process as outlined in Sect. 3.2 of this paper, which additionally provided two more products, namely, heavy oil and light oil, with some gases that were vented in the distillation column. As reported below, the product distribution after pyrolysis and further downstream processing (post-pyrolysis distillation) provides insight into the weight distribution of different components achieved through a continuous pyrolysis reactor.
After pyrolysis, the weight distribution for crude oil, char and gas is found to be 50%, 35% and 15%, respectively. Furthermore, following the distillation of the crude oil, the weight distribution was found to be 65%, 20% and 15% for heavy oil, light oil and light gases, respectively. The composition of each fraction is dependent on the pyrolysis conditions within the reactor and on the tyre composition as reported in Czajczyńska et al. (2017). Studies have been carried out to assess the effect of varying the reactor temperature and heating rate, which showed that by increasing the temperature within the reactor, the process resulted in a lower yield of char, with an increase in the yield of gaseous products. Generally, the process is optimized to increase the yield of liquid phase products due to the commercial utility of the product. Therefore, the study carried out here reports experimental data from our work—a crude oil composition of 50 wt% (liquid phase), char of 35 wt% (solid phase) and gas of 15 wt% (gas phase) —from the entire pyrolysis process.
Liquid phase
The liquid phase obtained from the pyrolysis process is known as pyrolysis oil, which is usually a dark and dense liquid, as shown in Supplementary Information Fig. S.6.
The continuous pyrolysis process produced a clean fuel that was not acidic in nature, as reported in Table S.7 in the supplementary information, summarizing the ultimate analysis performed on the crude oil, heavy oil and light oil (from the downstream processing). The oxygen content was calculated by a difference of approximately 0.4 wt%.
The absence of sulphur in these oils proves to be a significant result in this study with implications for its use. The components of this oil predominantly consist of aromatics such as xylenes, trimethylbenzenes, dimethyl styrenes and dimethyl indenes, accounting for nearly 65–79 wt%, as reported in previous studies Czajczyńska et al. (2017) and as observed through GC–MS analysis and comparing the peaks with the NIST database, as shown in Fig. 4. The light oil was observed to be extremely volatile in nature, and the calorific values of the oils are reported in Table S.7 at 45–48 Mj/kg.
Additionally, the GC–MS analysis shown in Fig. 2 conveys the carbon distribution information for the three oil samples that were analysed. Both the crude oil and post-distillation heavy oil correspond to diesel quality as reported in Wang et al. (2020) with carbon numbers C10-C24, whereas the light oil quality corresponds to that of petrol, C4-C9 (Shimdazu 2011).Footnote 3
Gas phase
The composition of the gas samples was analysed using micro-GC and is reported here within this section below. The gas obtained from the pyrolysis of waste tyres is known as pyrolytic gas, pyrogas or syngas as suggested in Czajczyńska, et al. (2017). The heating value of the pyrogas was calculated using the LHV formula and multiplying the heat of combustion values of the individual gases, both of which are available in the existing literature and are not presented within this paper. However, the calculations deemed the gas to be a highly combustible product of the pyrolysis process with a heating value of 36–39 MJ/kg. Consequently, it is quite obvious that the majority of the components in the pyrolytic gas are composed of hydrocarbons with four or fewer carbon atoms per molecule. Reviewing past studies, it has been suggested that these gases originate from the depolymerization process occurring in rubber components such as styrene-butadiene and the secondary cracking reactions that occur at higher temperatures. Comparing our results with those already published, similarities can be drawn towards the gas composition, where studies have reported predominantly hydrogen and methane (> 50 vol%) when the experiments were carried out in a nitrogen-free environment (Martínez et al. 2021), while a two-stage continuous process with in situ and ex situ desulphurization processes produced pyrogas with LHVs in the range of 38–43 MJ/kg as suggested in Choi et al. (2017).
-
Hydrogen: 8.5 mol% ± 10%
-
Methane: 27.7 mol% ± 10%
-
Ethane: 9.2 mol% ± 10%
-
Ethylene: 6.7 mol% ± 10%
-
Propane: 4.6 mol% ± 10%
-
Propylene: 6.6 mol% ± 10%
-
Butane: 2.2 mol% ± 10%
-
Butadyne: 34.5 mol% ± 10%
In contrast, Li et al. (2020) report that the composition of the pyrolytic gas includes a higher percentage of aromatic hydrocarbons over aliphatic compounds, which is possible depending on the process parameters such as temperature and gas residence time. Nevertheless, pyrolytic gas has a heating value that is close to that of natural gas and, therefore, is a promising fuel for present and future applications, with the current studies showing the promise of utilizing this as a source of fuel to sustain the pyrolysis process.
Solid phase
The pyrolysis solid residue is known as char or pyrolytic carbon black. In this particular study with the given operating parameters, approximately 35 wt% of solid residues were left over in the reactor following the pyrolysis process. A snippet of the char produced is provided in Supplementary Information Fig. S.10.
Generally, it has been reported by Czajczyńska et al. (2017); the residue is a mesoporous material having an average heating value of 30 MJ/kg: composed mainly of carbon and other inorganic compounds that were formed during the pyrolytic process. Therefore, to understand the porousness of the char material, a sample of residue was examined under a scanning electron microscope coupled with energy-dispersive spectroscopy and is shown in Supplementary Information Fig. S.11.
The solid residue is porous, soft and friable with a surface area of approximately 600 m2/gm, as obtained from the pyrolysis reactor. It is envisaged that physical activation with steam will further increase its surface area, potentially to 1000 m2/gm or higher. The composition of the bulk char samples was measured and reported in this paper, as provided in Supplementary Information Table S.12.
In addition to morphological and surface studies, energy-dispersive X-ray spectroscopy (EDX) was also carried out on samples of char particles (as opposed to the bulk composition shown in Table S.12, as shown in Fig. S.13 within the supplementary information provided). EDX analysis provides the surface composition at selected points on the char sample.
It can be inferred from the above analysis that the SEM–EDX composition is consistent with the bulk composition reported in Table S.12, validating the analysis to be accurate. Upon further investigation, the char sample was burnt to remove the remaining carbon to understand the mineral composition of the residue, as shown in Fig. S.14 within the supplementary information.
The residue generated (as shown in Fig. S.14 given in the supplementary information) was further subjected to SEM–EDX analysis, and it was observed that the carbon-free residue is a mineral-rich product mainly composed of zinc, silicon and some traces of bromine, which is reported in Fig. S.15 within the additional supplementary information provided. In an earlier study published by Martinez et al. (2019), the authors were able to demineralize the carbon black recovered from waste tyre pyrolysis, resulting in a product rich with carbon (92.9 wt%) and demonstrating its usability in SBR compounding, probing its utility as a substitute for commercial carbon black (N550), which is quite expensive.
Economic analysis of a 60 Tonne/day EOLT processing plant
The experimental results were collated to model a processing plant that has a capacity of 60 TPD to convert EOLT to fuels. The mass and energy balance is calculated on the basis of all experimental data presented in the previous sections. Based on the mass and energy balance presented in the process diagram, appropriate sizing was carried out to determine the required equipment for this processing plant. Thereafter, an economic analysis is performed to calculate the production cost of the products (diesel, char) to report the feasibility of a waste tyre pyrolysis plant in the context of the state of Victoria, Australia.
Process flow diagram with mass and energy balances
Based on the information generated in the experimental section, a process flow diagram of the EOLT pyrolysis facility including mass & energy balance is provided in Fig. 3.
As seen in Fig. 3, the final selling products consist of char and crude oil. The latter goes to a distillation column to produce heavy oil and light oil. The gases evolved during the pyrolysis stage are highly energetic (see Sect. 4.3.2) and can be utilized on-site for heating the pyrolyser as well as the distillation column.
Distillation of the heavy oil also produces very energetic light hydrocarbon gases, predominantly CH4 and C2H4, which have not been considered in the analysis. The char above can also be separated into carbon black to some extent. Table S.16 provided in the additional supplementary information presents the values of heat capacity, heat of decomposition, heat of evaporation of the volatiles and heat of distillation used for the energy balance.
Table S.17 and Table S.18 in the supplementary information present the energy balance for the pyrolyser. As shown in Table S.17, the net energy required for pyrolysis at 450 °C is approximately 48,000 MJ for 60 TPD of EOLT.
However, as reported in the TGA studies, the breakdown temperature of the waste shredded tyre initiates after 200 °C, and the maximum decomposition occurs at approximately 350 °C, with the breakdown terminating at 450–550 °C. Therefore, to start the pyrolysis process, external heat energy must be supplied to raise the feed’s temperature to at least that of the starting temperature required for decomposition (i.e., 200 °C). Then, the pyrolysis gas begins to build up within the reactor, and the external heating elements can be shut off, driving the pyrolysis process to an autothermal mode by combusting the gases generated in the process itself. As calculated from Table S.18, it can be concluded that approximately 30% of the energy required for the pyrolysis process should be drawn from an external source (either stored diesel from the process or gas burner) during start-up. At steady-state operation, approximately 63% of the pyrolytic gasses can be utilized to sustain the process.
The energy requirement for the distillation column was also calculated and is presented in Table S.18 within the additional supplementary information. Two distillation columns were considered for a 60 TPD plant. From the distillation point of view, the light gases evolving during the distillation phase are sufficient to sustain the energy required for the distillation column. Additional heat may be required to raise the temperature of the reactor materials and maintain the temperature, where the associated heat loss must also be considered. To account for this additional heat, 10% excess heat is considered in the total heat requirement.
Economic analysis
Based on the technical information (yield and composition of gas, yield of solid residues) generated in Sect. 4, an economic analysis was carried out for a 60 tonne per day steel-free waste tyre processing plant for a plant life of 20 years.
Before completing this task, a simple process model was developed using Aspen Plus and using the experimental data to examine whether the gas generated during pyrolysis has sufficient heat to sustain a pyrolysis process (with a conservative 10% extra heat considered to account for loss from the reactor) once the plant has started up from Cold start-up or Hot start-up. The results indicated that the processing plant can be self-sustaining during steady-state operation. As previously mentioned, tables in supplementary information provide more information on the energy balance.
Quotations were sought and received during our visit to suppliers and subsequent discussions over the phone. An economic spreadsheet model was developed to use the information generated from the experimental program and those obtained from suppliers. Apart from the base calculations, the sensitivity of the results to key operating parameters has been carried out, with the major products being gas, oil and carbon black. The generated gas has a high energy content and is assumed to be used for the pyrolysis process. We made eight assumptions to do the economic analysis. These assumptions are:
-
1.
The pyrolysis plant can process 60 TPD waste tyres into products. The plant works for 5 days a week that enable the processing of 15,600 TPY of waste tyres.
-
2.
The pyrolysis plant has a lifetime of 20 years. Plant depreciation (excluding the land cost) is considered a straight-line method, and 7 years is considered for complete depreciation.
-
3.
During the 1st and 2nd years, the plant runs at 80% and 90% of its capacity, respectively. After that, it runs at 95% of its capacity over the last 18 years
-
4.
After tyre pyrolysis, the product consists of 50% oil (crude), 35% char and 15% gases by mass. All the gases generated during the pyrolysis process are used within the pyrolizer. Externally purchased diesel is used for commissioning and cold start-up of the pyrolizer
-
5.
Among 35% of char, 20% is converted into carbon black. The crude oil is used for further distillation. We assumed the heavy oil to be sold as furnace oil at a price of $600/tonne. This assumption is justified by the heating value of the oil. The light oil is considered to be sold at $900/tonne, which is more likely diesel quality. The carbon black is assumed to be sold at $900/tonne which is lower than the
-
6.
The land purchased has to space for expanding the plant to the same capacity in the future. The selling cost of crude oil and carbon black is considered fixed over the entire plant life, a somewhat conservative estimate.
-
7.
All the running costs over the entire plant life are considered fixed
-
8.
The salary cost of the staff includes 80% overhead
The breakdown of the capital and operation costs is presented in Table 1.
Case scenarios
A sensitivity analysis was conducted to assess the comparative effect of input factors on the economic outcomes of the project.
The base case scenario is considered to be 100% land cost with no bank loan taken. All other operating costs and fixed costs are presented in Table 1 with the selling prices of the products given in Sect. 5.2.1.
Six scenarios are analysed, mainly changing the cost of land and the percentage of bank loans taken. In the calculation, the bank interest rate is considered 3% and remains fixed over the 20-year loan term.
Findings of economic analysis and sensitivity to major costs
The total fixed cost is estimated to be $11 M dollars; the land cost is a large part, 32%, of this fixed cost. Figure 4 (a, b) presents the cash flow analysis of the different scenarios considered. Without the land cost (land owned by the proponents), the cash flow is estimated to become positive in four years of operation. In the case of an 85% bank loan, the cash flow becomes positive after 6 years of plant operation.
Figure 4 (c, d) presents char and crude oil production costs for different scenarios. The production cost predominantly depends on the operating cost of the plant. The carbon black production cost varies between $225 and $375/tonne, depending on the assumptions. The crude oil production cost varies between 22 and 38 cents/litre depending on the assumptions. With an excise duty of 44.2 cents/litre, the oil price is considerably less than the current diesel price at the pumps of approximately $2/litre.
Figure 5 exhibits the calculated internal rate of return (IRR) and net positive value (NPV) after twenty years. The IRR varies from 15 to 35% depending on the amount of bank loan (0–100% take of the fixed capital cost) and land ownership (full ownership to complete purchase). The NPV varies from $80 M to $175 M for the above scenarios.
From the economic analysis, which is based on our own experimental results, communication with potential equipment suppliers and conservative estimates, it is evident that the pyrolysis process for processing waste tyres holds a significant upside in economic terms. This is in addition to the benefit of the zero landfill requirement of these wastes.
In the economic analysis, we assumed that among 35% of char, 20% was converted into carbon black.
Based on the morphology, EDX, physical characteristics, and chemical composition of the carbonaceous solid residues (see Sect. 4.3.3), the following uses, in no particular order, are recommended for further assessment and can improve the economics of the EOLT processing facility:
-
As solid fuel for gasification or combustion and then recovering Zn and Si from the noncarbonaceous residues
-
Use as catalyst or catalyst support in CO2 hydrogenation to chemicals using heterogeneous catalysis
-
A filler to modify asphalt, pigment for printing and substitute for commercial carbon black in rubber compounding
-
Production of graphene and other nanostructured materials and activated carbon for water treatment
Having said the above, a significant change in the interest rate or labour price will affect the economics. However, the principle of analysis remains the same. On the other hand, there may be environmental drivers that can fully or partially overturn economic considerations. These benefits are in addition to the benefit of the zero landfill requirement.
Sensitivity analysis of major cost parameters
Panels (a-d) in Fig. 4 and the two panels in Fig. 5 present results of varying the bank loan amount (of the total cost) on the payback period, production cost of char and oil, NPV and the IRR. As is evident, variation of the bank loan between 50 and 85% of the total cost does not have any significant effect on the payback period, and production cost of char and oil. However, there is a significant effect on the IRR and NPV. The IRR, however, remains acceptably high –15% to over 35%.
Further sensitivity analysis was done by varying the land cost (base case, 20% over base case) and the equipment cost (base case, 20% over base case) and varying the crude oil sale price at 600$/tonne and 800$/tonne. The effect of these variations on the IRR, NPV and payback period was calculated. The results are as follows:
-
Base case—with crude oil sale price $600/tonne: IRR 23.74%, NPV $156.2M, Payback period 5 years
-
20% increase in land cost holding equipment cost fixed as in base case with crude oil sale price $600/tonne: IRR 21.92%, NPV $146.8M, Payback period 6 years
-
40% increase in land cost holding equipment cost fixed as in base case with crude oil sale price $600/tonne: IRR 20.27%, NPV $137.4M, Payback period 6.3 years
-
20% increase in equipment cost holding land cost fixed as in base case with crude oil sale price $600/tonne: IRR 17.83%, NPV $119.4M, Payback period 7 years
-
40% increase in equipment cost holding land cost fixed as in base case with crude oil sale price $600/tonne: IRR 13.82%, NPV $82.7M, Payback period 8 years
-
20% increase in land cost holding equipment cost fixed as in base case with crude oil sale price $800/tonne: IRR 30.98%, NPV $229.6M, Payback period 5 years
-
40% increase in land cost holding equipment cost fixed as in base case with crude oil sale price $800/tonne: IRR 28.84%, NPV $220.2M, Payback period 5.1 years
-
20% increase in equipment cost holding land cost fixed as in base case with crude oil sale price $800/tonne: IRR 26%, NPV $202.2M, Payback period 5.3 years
-
40% increase in equipment cost holding land cost fixed as in base case with crude oil sale price $800/tonne: IRR 20.5%, NPV $165.5M, Payback period 6years
As is evident from the above, realistic increases in land or equipment costs significantly affect the IRR, NPV and the payback period. Having said that, all three financial parameters—IRR, NPV and payback period—remain acceptable.
Policy implications
Unlike waste plastics, tyres will continue to be produced and used throughout the world, generating end-of-life tyres. While a considerable number of EOLTs are generated in Australia, there is no processing plant in Australia to date. The generation sources are spread over the vast country. A dedicated supply chain needs to exist to make pyrolysis plants viable at defined locations. More importantly, defined regulatory policies in support of the pyrolysis process will facilitate the development of pyrolysis plants that are technically uncomplicated, as proven by our current experimental studies. The creation of a market to absorb the products from pyrolysis is essential. While being environmentally and technically feasible, the ability to use the three main derived products (liquid hydrocarbons, carbonaceous solids and excess noncondensable gases) defines the economic viability of the pyrolysis process.
Conclusions and further comments
Pyrolysis is one of the different processes for treating end-of-life tyres. Apart from energy generation from tyre-derived liquid fuel, material recovery, such as for use in road surfaces, flooring and new rubber products, has been proposed by many studies.
Pyrolysis represents a technically viable solution for processing significant volumes of end-of-life tyres and thereby reducing the unusable quantities from going to landfill. Such technology is used in parts of Asia, and similar large pilot-scale developments have been undertaken in Europe and North America. However, techno-economics and sustainability considerations for such technology are not well known.
Apart from the technical viability of producing good quality oil and carbon black, multiple other products are possible from the pyrolysis process. The oil is a good source of methane and hydrocarbons but not hydrogen or CO. Therefore, tyre-derived oil is not necessarily a good feedstock for chemicals.
Based on our experimental data, the techno-economic analysis, a conservative conclusion of 20% diesel, ~ 65% furnace oil and 7% carbon black, shows a cash-flow positivity for a 60 TPD processing plant under five years. Distillation of the generated crude oil was carried out at 230 °C. Further optimization of the operating conditions and distillation temperature is possible; these are likely to improve the techno-economics of the process further.
Pyrolysis in the current study was carried out at a constant temperature of 450 °C. Considerations for higher temperatures will likely produce more gas and less oil. The yield of char will mostly remain constant. Based on the experimental and economic analysis data, we believe that the adoption of the pyrolysis process will solve the problem of stockpiling or landfilling of end-of-life tyres.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
Notes
Refer Green Distillation Technologies Corporation Ltd (2020), Cleanly Recycled Tyres—A World Technology First, [Online]. Available: https://www.gdtc6.com/, and Pearl Global 2020. [Online]. Available: https://pearlglobal.com.au/. [Accessed 2 December 2022].
Model STA 449F3, NETZSCH, Selb, Germany.
Shimdazu, Application data sheet No.21, 2011. [Online]. Available: https://www.ssi.shimadzu.com/sites/ssi.shimadzu.com/files/Products/literature/GCMS/021_gcms_datasheet.pdf. [Accessed 1 December 2021]. Refer, supplementary information S.8 and S.9.
References
Arabiourrutia M, Lopez G, Artetxe M, Alvarez J, Bilbao J, Olazar M (2020) Waste tyre valorization by catalytic pyrolysis—A review. Renew Sustain Energy Rev 129:109932
Auxilio AR, Choo W-L, Kohli I, Srivatsa SC, Bhattacharya S (2017) An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manage 67:143–154
Aylón E, Callén M, Lopez JM, Mastral AM, Murillo R, Navarro MV, Stelmach S (2005) Assessment of tire devolatilization kinetics. J Anal Appl Pyrol 74(1–2):259–264
Choi G-G, Oh S-J, Kim J-S (2017) Clean pyrolysis oil from a continuous two-stage pyrolysis of scrap tires using in-situ and ex-situ desulfurization. Energy 141:2234–2241
Czajczyńska D, Krzyżyńska R, Jouhara H, Spencer N (2017) Use of pyrolytic gas from waste tire as a fuel: A review. Energy 134:1121–1131
Galvagno S, Casu S, Casabianca T, Calabrese A, Cornacchia G (2002) Pyrolysis process for the treatment of scrap tyres: preliminary experimental results. Waste Manage 22(8):917–923
Genever K, O’Farrell P, Randell, Rebbechi J (2017) National market development strategy for used tyres: final strategy. Tyre Stewardship Australia
Ghodrat M, Alonso JA, Hagare D, Yang R, Samali B (2019) Economic feasibility of energy recovery from waste plastic using pyrolysis technology: an Australian perspective. Int J Environ Sci Technol 16:3721–3734
Pearl Global, 2020. [Online]. Available: https://pearlglobal.com.au/. [Accessed 2 December 2021].
Green Distillation Technologies Corporation LTD, “Cleanly Recycled Tyres—A World Technology First,” 2020. [Online]. Available: https://www.gdtc6.com/. [Accessed 2 December 2021]
Kumaravel S, Murugesan A, Kumaravel A (2016) Tyre pyrolysis oil as an alternative fuel for diesel engines—A review. Renew Sustain Energy Rev 60:1678–1685
Li D, Lei S, Lin F, Zhong L, Ma W, Chen G (2020) Study of scrap tires pyrolysis- Products distribution and mechanism. Energy 213:119038
Martínez JD, Puy N, Murillo R, García T, Navarro MV, Mastral AM (2013) Waste tyre pyrolysis—A review. Renew Sustain Energy Rev 23:179–213
Martínez JD, Cardona-Uribe N, Murillo R, García T, López JM (2019) Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manage 85:574–584
Martínez JD, Campuzano F, Cardona-Uribe N, Arenas CN, Muñoz-Lopera D (2020) Waste tire valorization by intermediate pyrolysis using a continuous twin-auger reactor: Operational features. Waste Manage 113:404–412
Martínez JD, Campuzano F, Agudelo AF, Cardona-Uribe N, Arenas CN (2021) Chemical recycling of end-of-life tires by intermediate pyrolysis using a twin-auger reactor: Validation in a laboratory environment. J Anal Appl Pyrol 159:105298
Martinez JD (2021) An overview of the end-of-life tires status in some Latin American countries : Proposing pyrolysis for circular economy. Renew Sustain Energy Rev 144(2021):111032
Seidelt S, Müller-Hagedorn M, Bockhorn H (2006) Description of tire pyrolysis by thermal degradation behaviour of main components. J Anal Appl Pyrol 75(1):11–18
Shimdazu, “Application data sheet No.21,” 2011. [Online]. Available: https://www.ssi.shimadzu.com/sites/ssi.shimadzu.com/files/Products/literature/GCMS/021_gcms_datasheet.pdf. [Accessed 1 December 2021].
Wang X, Wang M, Han Y, Chen H (2020) Identifying unregulated emissions from conventional diesel self-ignition and PPCI marine engines at full load conditions. J Marine Sci Eng 8(2):101
“Tyre Stewardship Australia,” 29 November 2021. [Online]. Available: https://www.tyrestewardship.org.au/about-tsa/.
Wey M-Y, Liou B-H, Wu S-Y, Zhang C-H (1995) The Autothermal Pyrolysis of Waste Tires. J Air Waste Manag Assoc 45:855–863
Williams PT, Besler S (1995) Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 74(9):1277–1283
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work is partly funded by Monash University.
Author information
Authors and Affiliations
Contributions
MA Kibria and BS Thomas completed the experiments and sample analysis. BM Kibria wrote the first draft and also completed the process simulation and economic analysis. Mita Bhattacharya formulated the project, reviewed the economic analysis, formulated the policy prescription and reviewed the manuscript. Sankar Bhattacharya formulated the project, reviewed the process simulation, completed the economic analysis and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kibria, M.A., Thomas, B.S., Bhattacharya, M. et al. Processing of metal-free end-of-life tyres (EOLTs) to fuels and products: an experimental study with process simulation and economic analysis from an Australian perspective. Clean Techn Environ Policy (2024). https://doi.org/10.1007/s10098-024-02825-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10098-024-02825-y