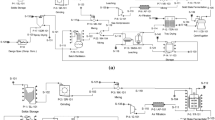
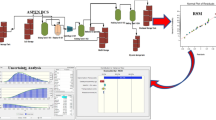
Abstract
This study aims to propose a new process design, simulation, and techno-economic analysis of an integrated process plant that produces glucose and furfural from palm oil empty fruit bunches (EFB). In this work, an Aspen Plus-based simulation has been established to develop a process flow diagram of co-production of glucose and furfural along with the mass and energy balances. The plant’s economics are analyzed by calculating the fixed capital income (FCI), operating costs, and working capital. In contrast, profitability is determined using cumulative cash flow (CCF), net present value (NPV), and internal rate of return (IRR). The findings show that the production capacity of 10 kilotons per year (ktpy) of glucose and 4.96 ktpy of furfural with a purity of 98.21 and 99.54%—weight, respectively, was achieved in this study. The FCI is calculated as United States Dollar (USD) 20.80 million, while the working and operating expenses are calculated as USD 3.74 million and USD 16.93 million, respectively. This project achieves USD 7.65 million NPV with a positive IRR of 14.25% and a return on investment (ROI) of 22.06%. The present work successfully develops a profitable integrated process plant that is established with future upscaling parameters and key cost drivers. The findings provided in this work offer a platform and motivation for future research on integrated plants in the food, environment, and energy nexus with the co-location principle.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The usage of natural resources has spiked up rapidly due to the tremendous increase in the global population. The vast number of resources consumed generally results in more waste. It is anticipated that by 2050, worldwide municipal solid waste (MSW) will be over 3.40 billion metric tonnes (MT) (Ellis 2018). Food waste dominated the highest percentage, followed by paper and paperboard, plastic, yard trimmings, and metals (Aydin 2015; Aydin et al. 2017; Celep et al. 2013; The World Bank 2021). These wastes are usually disposed of in landfills, recycled, composted, or utilized in energy recovery.
Malaysia, Indonesia, and Thailand are the leading producers of palm oil, accounting for more than 90.00% of the global market in total, with Malaysia accounting for 25.90% of global production and 33.70% of global palm oil exports in 2019 (MPCO 2021; Zafar 2021). In a palm oil mill, fresh fruit bunch is employed as a raw material; however, only 23% of the raw material is utilized to make palm oil, with the remainder being trash (Aziz et al. 2015). There is an abundance of waste being produced from palm oil plantations or mills, such as palm oil empty fruit bunch (EFB), fibers, and nutshells, which require processing into value-added products or undergo waste minimization processes (Aziz et al. 2015). In certain scenarios, open-ended burning is used to dispose of EFB trash, causing huge environmental difficulties, including global warming potential (GWP) and air pollution (Ninduangdee and Kuprianov 2016). These EFB can also be reused as biomass, reducing the processing plant's economic and environmental burdens. Since EFB biomass is low in sulfur, it reduces GWP and air pollution (Ninduangdee and Kuprianov 2016). Furthermore, EFB can be processed into high-value products such as glucose, xylitol, levulinic acid, and vanillin (Hafyan et al. 2020; Umana et al. 2020). In addition, it can be chemically processed into ethanol, furfural, and lactic acid and converted into energy such as bio-oil, biogas, bioethanol, and methane (Geng 2013).
Malaysia, Indonesia, and Thailand generated 27 million MT of EFB in 2019 (Zafar 2021). Since EFB is readily available in Malaysia, it is an inexpensive renewable energy source (Alaw and Sulaiman 2019). These EFB can be further processed to extract valuable food and chemicals; hence, promoting resource conservation and sustainable processes (Alaw and Sulaiman 2019). However, there is still much waste to date, even if many plants use the EFB to manufacture foods, chemicals, or energy (Chiew and Shimada 2013). This is because the existing process plant only valorizes a particular component in EFB such as cellulose or hemicellulose only. This procedure also requires many separations, increasing the capital cost. To overcome this, lignocellulosic biomass can be processed into numerous products in a single plant in an integrated way (Jin et al. 2018; Orugba et al. 2021). For an integrated process plant producing both food and chemicals, glucose and furfural are selected as end products because they can be produced in a single facility with minimal equipment (Buaisha et al. 2020; Loginova et al. 2021). Moreover, the residual lignin can be used as a fuel for boilers to supply heat energy. Hence, the plant's economic and environmental impacts are reduced as a result of maximizing EFB's potential.
Currently, only a few studies have worked on an integrated process plant employing hemicellulose in biomass. Loginova et al. (2021) developed an integrated system to convert different lignocellulosic biomass (i.e., wheat straw, corn cobs, sugar beet pulp, birch sawdust, and oat husk) to furfural and glucose. In addition, an integrated biorefinery process for co-production of glucose and xylose (i.e., raw material for producing furfural) from wheat straw has been developed and analyzed by Liu et al. (2021). Moreover, Choi et al. (2019) proposed a simultaneous synthesis process of glucose, ethanol organosolv lignin, and furfural from lignocellulosic biomass. Although their system can produce glucose and xylose or furfural, their study only focused on experimental work and mass balance calculation without sufficient techno-economic analysis.
In addition, those previous studies are not solely dedicated to converting EFB to glucose and furfural. Also, the process conditions were not optimized to maximize the production rate and purity. Existing research also lacks modeling data and production rates for integrated processes. The process design and optimization can also assist in achieving the requisite product purity. Moreover, existing work lacks economic analysis and profitability to assess an integrated plant's viability. Therefore, this work aims to use simulation to obtain the mass and energy balance for optimizing the integrated process and perform an economic analysis to identify the overall profitability and plant viability.
In an integrated production process, maximizing feedstock utilization by producing multiple outputs is crucial in order to increase the efficiency of feedstock consumption. Recent research has revealed that if two industries were co-located, there would be opportunities for major resource sharing, notably in the area of energy (Ahmad et al. 2019; Solomou et al. 2022). In this regard, Sheppard et al. (2019) also emphasize the mutual benefits that could result from the co-location of biorefineries and food and beverage manufacturing facilities, such as enhancing the material and energy flows between the two facilities, reducing manufacturing costs, as well as integrating heat and water consumption more effectively than a single facility. Hence, integrated production of furfural and glucose has the potential to demonstrate substantial economic advantages and returns.
The significance of this study is first to address the techno-economic feasibility of an integrated plant that can co-produce food and chemicals using EFB biomass. In this regard, the food safety aspect is indeed important. In the latter stage, cleaning in place (CIP) and hazard analysis critical control points (HACCP) practices that can sterilize the process facilities will be implemented for glucose production to ensure the process is in compliance with food production, safety and hygiene standards (Banach et al. 2020; Chaib and Barone 2020; Malliaroudaki et al. 2022; Motarjemi and Lelieveld 2013). By practicing the CIP and HACCP principles, the proposed model of glucose and furfural co-production can concurrently optimize the planning, design, and operation of value chains for EFB biomass with integrated food and chemical production.
Converting EFB components into value-added products reduces waste generation and promotes a circular economy. Using process simulation of Aspen Plus (Aspen Technology Inc.), the production rate, purity, and flowrate of the product can be calculated and adjusted to reduce raw material consumption and eventually reduce the capital cost. When evaluating the profitability of the integrated plant, it helps to compare the main cost drivers of the integrated plant. Furthermore, sensitivity analyses optimize production parameters such as capacity and fixed capital. Overall, the plant viability can be determined by the process sustainability and profitability.
Process description
Figure 1 shows the research flowchart of this study. First of all, the conceptual process design based on the characteristics of the raw material and the requirements of the product (i.e., purity) has been proposed. The output of the process design leads to the generation of a block flow diagram and process flow diagram (PFD). To determine the viability of a proposed plant, unit operations were arranged in series to form a flowsheet that can be simulated in Aspen Plus. Some parameters are manipulated in techno-sensitivity analyses to obtain the optimum operating conditions. The mass and energy balances generated from the Aspen Plus simulation become the basis for analyzing the plant’s capital and operating costs before conducting economic sensitivity analyses. Following this, a comprehensive techno-economic analysis determines the plant's viability. In addition, the findings are analyzed by comparing them with other literature reviews that produce furfural and glucose and with other existing commercial plants.
Raw material and products
Palm oil empty fruit bunch
EFB is lignocellulosic biomass composed of cellulose, hemicellulose, and lignin where it can be broken down into simple sugars to produce various food additives (Geng 2013). On the other hand, this biomass can also be converted to renewable energy such as syngas, ethanol, furfural, and bio-oil through thermochemical or biological conversion (Geng 2013) (Table 1).
Furfural
Furfural is an oily, colorless liquid that turns dark brown when exposed to air (Ebert 2008). It is used to refine lubricating oil, as a fungicide in tetrahydrofuran manufacturing, and as an industrial solvent (Ebert 2008). Moreover, it is an important raw material for the production of furfuryl alcohol by catalytic hydrogenation and is also used for other transportation fuels like ethyl levulinate and dimethylfuran (Ebert 2008; Mordor Intelligence 2021). Furfural is produced at high purity with low moisture content by dehydration of 5-carbon sugar xylose and arabinose (Ebert 2008). Table 2 provides its specifications.
In 2018, worldwide furfural exports and imports increased to 146.70 and 155.70 kilotons per year (ktpy), respectively (UN Data 2018). In Malaysia, the import demand for furfural reached 267.80 tpy in 2018 (UN Data 2018), and this value is expected to with a compound annual growth rate (CARG) of 5% by 2026 (Mordor Intelligence 2021). Among the global consumers, China is the largest consumer globally, followed by western Europe and other Asian countries (IHS Markit 2020).
Glucose
Glucose is a viscous, colorless, and sweet fluid that improves the food texture, taste, and gloss (Wilson and Lilly 1969). Therefore, it is often used as a sweetener in the production of food and chemicals industries (Basso and Serban 2020). Glucose can also be converted into bioethanol through fermentation with yeast, producing bioenergy, or into levulinic acid, succinic acid, lactic acid, etc. (Hafyan et al. 2020). Industrial glucose production comes in various compositions and purity, which can be benchmarked by their dextrose equivalent (DE) value, which measures the percentage of inverted sugar, including glucose, oligomer, dextrose, and maltose (Sarungalo 2005). The sweetness of glucose syrup is determined by the feedstock's DE content, including low DE (26–29%), high DE (40–45%), and the sweetest DE (56–64%) (Sarungalo 2005). For EFB, the DE falls between 42 and 45%, and the composition is shown in Table 3 (21Food 2021).
In 2018, worldwide glucose exports and imports increased to 2995.50 and 3773.30 ktpy, respectively (UN Data 2018). In Malaysia, the import demand for glucose reached 44.46 tpy in 2018 (UN Data 2018), and this value is expected to double by 2030, with a compound annual growth rate (CARG) of 5.20% (Persistence Market Research 2020). Belgium is the worlds’ largest consumer, whereas Malaysia stands in 13th place (UN Data 2018).
Process flowsheet of the integrated process
The glucose and furfural are produced via enzymatic hydrolysis and dehydration, respectively. The process includes breaking down the EFB to separate cellulose and hemicellulose in feed preparation. Furthermore, it is followed by enzymatic hydrolysis for glucose production and dehydration for furfural synthesis along with purification and recovery of the products to achieve the desired specification. Figure 2 shows the associated process description of each unit.
Feed preparation
The EFB is purchased from the palm oil processing plant and stored in a warehouse before being conveyed to the feed preparation. Initially, the EFB contains 19.8 wt% moisture, which is reduced to 10 wt% using 105 °C hot air in the rotary dryer (S-1001) (Loh 2018). Then, the dried EFB is transported to a grinder (S-1002) to be cut into small pieces with a size between 0.10 and 1.00 mm (Kenthorai Raman and Gnansounou 2015). Furthermore, it is transported to a vibrating screen (S-1003), which contains an 80–20 wired mesh to separate the smaller particles sized below 1 mm. In contrast, the larger particles are recycled back to the grinder (S-1002) to obtain uniformly sized EFB (Kenthorai Raman and Gnansounou 2015).
Pretreatment
In the pretreatment section, process water and 70 wt% dilute sulfuric acid are pumped from its storage tank to an agitated mixing tank (M-1101) operating at 30 °C and 1 atm. Simultaneously, the recycled water from the furfural purification unit (C-1302) is pumped to the mixing tank (M-1101). Here, the sulfuric acid is diluted using the process and recycled water and then mixed with the ground EFB from the feed preparation unit (CONV-1101) in an agitated mixing tank (M-1102). The flowrate of sulfuric acid and process water is adjusted to 30% of solid loading with 18 mg of sulfuric acid per g of dry EFB (Humbird et al. 2011). The mixture is then pumped (P-1104) to 6 atm and heated (E-1101) to 158 °C before being fed into an insulated continuous stirred tank reactor (CSTR) (R-1101) and stirred for 5 min (Humbird et al. 2011). The reactor converts most hemicellulose to xylose, with secondary reactions as shown in Table 4.
The product from CSTR (R-1101) is cooled (E-1102) to 50 °C for solid separation. The cooled product is sent to a decanter centrifuge (S-1101) to separate solid and liquid. The decanter centrifuge can separate particles sized from 0.10 to 1.00 mm using its centrifugal force rotating horizontally, separating the solids on the separator wall, and removing them using a screw conveyor (Dolphin Centrifuge 2021). The solid is completely removed with 5 wt% moisture and sent to the neutralization unit, while the liquid stream is sent to the dehydration unit.
Furfural synthesis
The liquid stream from the pretreatment is dehydrated to convert xylose into furfural, where the reaction is autocatalyzed by heat. Firstly, the liquid is heated to 170 °C and pumped (P-1201) to 8.80 atm into an insulated CSTR (R-1201) (Mittal et al. 2017). The residence time of the reaction is 20 min. As our processing plant is continuous, the CSTR (R-1201) has a 3.62 m3 capacity, accounting for the residence time. The reactors were assumed to have a maximum operating capacity of 80% of the designed volume (Mittal et al. 2017). In CSTR (R-1201), the conversion of xylose into furfural can be achieved up to 96.50% (Mittal et al. 2017).
Furfural recovery
The furfural mixture from the dehydration unit requires further purification because it contains water, sulfuric acid, and acetic acid, which must be removed to obtain high purity of furfural. Initially, the condensed liquid from the dehydration unit is fed into a distillation column (C-1301), where furfural and water are recovered as distillate (top) and the bottom stream, which contains diluted acid from the distillation column. The bottom stream is directed to the wastewater treatment unit. Meanwhile, the distillate is cooled (E-1303) to 40 °C before it is diverted to a liquid–liquid extraction column (C-1302), where butyl chlorine is introduced as a solvent to break up the azeotrope of furfural and water (Nhien et al. 2021). Fresh butyl chloride from its storage tank is mixed with the recycled butyl chloride from the distillation column (C-1303) in a mixer (M-1301) and heated (E-1304) to 40 °C (Nhien et al. 2021). The heated solvent is fed to the extraction column (C-1302), where the water is separated in the bottom stream, recycled to the pretreatment unit, and 5 wt% of water is purged to avoid accumulation in the production system (Nhien et al. 2021). The furfural-butyl chloride stream from the top is sent to the second distillation column (C-1303) for solvent recovery (Nhien et al. 2021). The furfural-butyl chloride mixture is separated using a distillation column (C-1303), where the purity of furfural is achieved to a value of 99.54 wt%. The purified furfural (bottom) is cooled to 30 °C, which is then transported to the storage tank; the remaining solvent is left as a distillate from the distillation column for recycling.
Glucose synthesis
Glucose synthesis is separated into two units, i.e., neutralization and saccharification units. The wet solid stream from pretreatment is fed into an agitated mixing tank (M-1402). The diluted base (neutralizing liquid), which contains 50 wt% of sodium hydroxide, process water, and the recycled water from the glucose purification unit (C-1601), are pre-mixed in an agitated mixing tank (M-1401) before being further mixed in M-1402 (Humbird et al. 2011). The flowrate of sodium hydroxide is fed such that it is equimolar to the moles of sulfuric acid and acetic acid in a wet solid stream while the process water flowrate is adjusted to account for 20% solid loading in M-1402 (Humbird et al. 2011). To account for complete neutralization of acetic acid, sodium hydroxide is fed 10 mol% in excess (Humbird et al. 2011). In this reaction, sulfuric acid and acetic acid are converted into sodium-based salt (e.g., sodium sulfate and sodium acetate). After neutralization of acids, the pH of the slurry is increased to pH 5, and it is further heated (E-1401) to 48 °C (Humbird et al. 2011).
In the saccharification unit, the heated slurry from the neutralization unit is fed into an insulated CSTR (R-1501), which operates at 48 °C and 1 atm (Humbird et al. 2011). Cellulase enzyme from its storage tank is fed into the CSTR (R-1501) (Humbird et al. 2011). With enzymatic hydrolysis, 95.20 wt% of the cellulose is converted into glucose (Humbird et al. 2011). The residence time of the reaction is two successive days (48 h). The slurry from CSTR (R-1501) is pumped into a decanter centrifuge where all the solids that contain 5 wt% moisture are removed and sent to the boiler feed unit as fuel stock while the liquid stream is sent to the final stage for glucose purification.
Glucose recovery
The glucose mixture from the saccharification unit contains glucose, water, and other impurities. For glucose purification, the glucose mixture from the saccharification unit is fed into a distillation column (C-1601), where most glucose can be separated in the bottom stream. The distillation column contains four stages with a reflux ratio of 1, and the feed is at the second stage from the top. This separation method increases glucose purity to 96.67 wt%. The remaining water is removed as a distillate from the distillation column and chilled (E-1603) to 30 °C before being recycled to the neutralization unit with a 5% purge rate. Finally, the glucose stream is cooled (E-1604) to 30 °C and pumped into the glucose storage tank.
Process simulation
Aspen Plus features a variety of modules and thermodynamic databases for simulating chemical processes. Therefore, the integrated process of glucose and furfural production from EFB (Fig. 3) is simulated using Aspen Plus V10, with the process conditions obtained from the literature review (Dolphin Centrifuge 2021; Humbird et al. 2011; Kenthorai Raman and Gnansounou 2015; Loh 2018; Mittal et al. 2017; Nhien et al. 2021). Here, the feed preparation unit in Fig. 3 is not included in the process simulation since Aspen Plus limits the usage of solid handling and batch processes. Therefore, it is assumed that the EFB is dried and ground. In some major unit-operation, there is a lack of information on equipment design and operating parameters. Hence, the following assumptions are made in the present work:
-
The process is continuous and in a steady state.
-
There is no loss of pressure and temperature across equipment and pipelines.
-
All transfer pumps increase by 0.1 atm to compensate for pressure loss in the pipeline.
-
Positive displacement pumps are used for streams with a capacity lower than 0.55 m3/h and streams with solid–liquid slurry (Liquiflo 2016), while others rely on centrifugal pumps (Liquiflo 2016).
Set up of Aspen simulation
Property method
This study employed NRTL as the thermodynamic property package, which is identical to the existing studies in literature (Humbird et al. 2011). However, the binary interaction between certain components such as butyl chloride and furfural was not included in the NRTL; therefore, UNIQUAC property package can be used for vapor–liquid equilibrium (VLE) as well as liquid–liquid extraction (LLE) (Manual 2001). Thus, NRTL is chosen as the base property method while UNIQUAC property method is added to the referenced method in Aspen Plus.
Defining components
All liquid components are available in the Aspen database. However, the solid component, i.e., EFB; however, is not available in the Aspen database. Therefore, it has to be added manually by defining the corresponding properties such as molecular weight, solid enthalpy of formation, solid molar volume (VSPOLY-1), and solid head capacity (CPSPO1-1). This information of which can be obtained from the literature (Wooley and Putsche 1996).
Sensitivity analyses
Sensitivity analyses are conducted to maximize the yield and purity of furfural and glucose in the product purification units. The influence of 4 manipulated variables is examined in order to determine the optimum operating conditions. Two-point values are applied to examine the influence of the higher and lower bounds on the parameters to which the yield and purity of products are most sensitive. As Aspen Plus has calculated the binary interaction between the molecules, the reflux ratio, and a number of stages in the distillation column are varied to compare the purity and flowrate of the product. In addition, a solvent used in the liquid–liquid extraction column and the purge percentage of both recycled streams are varied to determine the most suitable recycle flowrate and save cost.
Number of stages
Figure 4 shows the product composition in the distillation columns 1 to 3 and the liquid–liquid extraction column. The number of stages of distillation columns 1 and 2 is varied from 5 to 15. Based on Fig. 4, distillation column 1 gives the highest purity and flowrate of furfural at Stage 10th; distillation column 2 achieves the maximum purity and flowrate of furfural at Stage 13th. On the other hand, the number of stages of the final distillation column ranges from 2 to 10. The maximum flowrate and purity of glucose were found to be 34.86 kg/h and 98.18 wt%, respectively, at the 3rd stage before the flowrate and purity declined slightly to 34.86 kg/h and 98.15 wt%, respectively. For the LLE column, the optimum stage was found to be the 7th stage. From Fig. 4, it is apparent that the number of stages only provides a marginal effect on the furfural flowrate and purity. Therefore, it is not recommended to increase the number of stages as the marginal increase in the furfural flowrate and purity were achieved at the expense of an increase in the column capital cost.
Reflux ratio
Figure 5 shows the effect of the reflux ratio on the composition and yield of products. The reflux ratio in all three distillation columns was varied from 0.7 to 1.5. Based on Fig. 5, distillation column 1 gives the highest purity and flowrate of furfural at a 1.5 reflux ratio. For distillation column 2, the optimum reflux ratio is observed at the intersection point between both lines, which is 0.9. For distillation column 3, the increase in reflux ratio has no significant effect on the flowrate and purity of products; thus, original reflux ratio of 1 is used. Overall, the optimal reflux ratio is recorded at 1.5 for distillation column 1 and 0.9 for distillation column 2. Meanwhile, the reflux ratio of distillation column 3 remains at 1, based on the original literature review data (Nhien et al. 2021).
Solvent type
The type of solvent is analyzed in a liquid–liquid extraction column (C-1302) between butyl chloride and toluene. According to Nhien et al. (2021), the feed-to-solvent ratio for furfural is lower for toluene at 2.68, whereas butyl chloride has a feed-to-solvent ratio of 7.68. From process simulation using Aspen Plus, the purity and flowrate of furfural were studied for both solvents, and the result is recorded in Table 5. Based on the table, butyl chloride and toluene solvents produce similar furfural purity (99.98 and 99.50 wt%, respectively) and flowrate (17.20 and 17.27 kg/h, respectively). Thus, both are promising solvents, and a literature review is performed to select the most suitable solvent. Although a higher amount of butyl chloride is needed, as mentioned previously, the amount of energy required in the butyl chloride case is less than that in the toluene case (Nhien et al. 2017). With this regard, Nhien et al. showed that butyl chloride is the best solvent for the furfural manufacturing process, saving 44.70% of the total annual cost while lowering CO2 emissions by 45.50%, in comparison to using toluene and benzene as solvents. Therefore, the plant expenses can be saved. The selling price of furfural can be increased due to its higher grade and purity when butyl chloride is selected as a solvent in the furfural production unit.
Recycle flow rate
To avoid the accumulation of undesirable chemicals in the process as a result of recycling, some parts of the recycling streams are purged before being recycled back into the process. In this sensitivity analysis, the first recycle stream (Recycle 1) consists of 99.5 wt% water, 0.3 wt% acetic acid, 0.1 wt% butyl chloride, and 0.1 wt% furfural, while the second recycle stream (Recycle 2) mainly consists of water (99.90 wt%) with 0.10 wt% of furfural as an impurity. Figure 6 shows the effect of purge fraction on the flowrate and purity of products. The purge percentage for both Recycle 1 and 2 is varied from 0 to 10%. The first recycle stream with 99.50 wt% of water recycled back into pretreatment unit achieves the optimum flowrate and purity of furfural (17.53 kg/h and 99.54 wt%, respectively) at a 5% purge percentage. Based on Fig. 6, the flowrate of the furfural is inversely proportional to the purge percentage, while the purity of the furfural is directly proportional to the purge percentage. In this aspect, decreasing the mass flowrate of recycling streams improve the purity of furfural. Due to minor improvement in flowrate and a slight decrease in purity of furfural, the optimum purge percentage is recorded at the intersection point between both lines. The second recycling stream which recycles 99.90 wt% of water back to the neutralization unit, has an optimum glucose purity and flow rate (34.86 kg/h and 98.25 wt%) at a purge percentage of 10%. In this case, the purge fraction has no significant effect on the glucose flow rate while the purity of the glucose increases with increasing purge percentage. Overall, 95% and 90% of water is recovered and recycled back to the pretreatment and neutralization units for reuse purposes, respectively.
Simulation results
The optimization results are recorded in Tables 6 and 7. Based on the integrated process of glucose and furfural production from EFB, the desired final product purity is obtained using a basis of 100 kg/h of dry EFB. After optimization, the final purity and flowrate of the product are achieved with the absence of cellulase enzymes in the final product. Further, the process is optimized by changing the equipment design based on optimum operating conditions to maximize the purity and flowrate of final products. Therefore, optimized simulation attains better plant efficiency and minimizes waste produced. After optimization, the mass flowrate is scaled by adjusting the feedstock (dry EFB) to attain the annual plant capacity (i.e., 10 ktpy of glucose and 4.96 ktpy of furfural), where the plant can achieve a purity of 98.21% for glucose and 99.54% for furfural. Besides the purity and flowrate of products, the overall heating duty of the plant is reduced to 3385.91 kW, whereas the overall cooling duty is reduced to 2247.39 kW after optimization. According to Table 7, the amount of heating and cooling duties is reduced by 0.27% and 0.75%, respectively, which can minimize energy consumption and production costs in the economic analysis.
The experimental work of Montané et al. (2002) revealed that the hydrolysis of lignocellulosic biomass with H2SO4 yields a range of 50 to 65% of furfural. Similarly, the experimental work of Jung et al. (2013) revealed that 88.5% of glucose yield was determined after 48 h of the hydrolysis process. Both furfural and glucose yields (67.59% and 94.45%, respectively) in this study are higher than the findings in the experimental work. Meanwhile, the furfural and glucose purities (99.54 wt% and 98.21 wt%, respectively) obtained from the optimized Aspen Plus simulation were indeed higher than those of the commercially available furfural (98 wt%) and glucose (98 wt%) (Fisher Scientific 2022; Sigma-Aldrich 2022), thus demonstrating the feasibility of the proposed process design.
Economic analysis
For economic analysis, the total capital investment is calculated using all the equipment costs according to its capacities and flowrate of feedstock, product, and utility costs. The summary of feedstock, product, and utilities is shown in Table 8.
Fixed capital investment
Fixed capital investment (FCI) is calculated to build a new plant, including the building, construction, and equipment costs required. The fixed capital is calculated using equipment prices from 2006 to 2002 adjusted to inflation and location (Peters et al. 2003; Sinnott and Towler 2020). The total fixed capital investment was found to be United States Dollar (USD) 20.80 million. Among them, the inside battery limit (IBL) cost alone covers 48% of the FCI as the plant uses various types and numbers of equipment.
Direct cost and indirect cost
Direct cost can be divided into IBL and outside battery limit (OBL). IBL is the total cost of purchasing and installing all process equipment inside the process boundary; OBL is the total cost required for building and yard improvements along with the construction and installation of utility systems, storage, and waste treatment systems that are outside the process boundary.
IBL costs are adjusted along with installation, electrical system, control system, phase handle, and piping cost within the process boundary. The cost of equipment is determined from Chemical Engineering Design by Gavin and Sinnott at U.S. Gulf in 2006 and Plant Design and Economics for Chemical Engineers by Peters in the USA in 2002 (Peters et al. 2003; Sinnott and Towler 2020). Finally, the cost of each piece of equipment can be calculated by adjusting the location factor to Malaysia, cost indices to 2020, and its Lang factor accordingly.
In order to cover the expenses on food production safety procedures, 15% of additional equipment costs were assumed in the glucose production units. Remarkably, the 15% increase in equipment costs has covered various essential food safety factors, such as a) cleaning agents and disinfectants for CIP, b) extra pump work and water needed for washing, flushing, and rinsing, as well as c) water that meets food safety standards, which can be purchased from portable water production (Moerman et al. 2014). Besides that, sterilization is accomplished in the pretreatment unit using the heat treatment technique, with the stream being heated to 158 °C using E-1101 and cooled to the saccharification temperature of 30 °C using E-1102 before entering the glucose production units (Pina et al. 2014). In this study, hot pressure steam was selected for the sterilization since other sterilization methods such as gamma rays and ethylene oxide gas might prevent polymerization after repeated irradiation and cause potential toxicity (Lightfoot and Maier 1998). Moreover, glucose was recovered at 110.18 °C as the final product from the distillate stream of the distillation column (C-1601), exceeding the minimum sterilizing temperature (110 °C). In this case, microorganisms such as coliforms, lactic acid bacteria, and Bacillus cannot withstand temperatures above 110 °C (Błaszczyk and Iciek 2021).
There are a lot of critical parameters that need to be controlled in this study, such as (a) temperature and (b) pressure of the condenser or reboiler on the distillation column as well as (c) product composition at the outlet of the distillation column in the product recovery units. In order to achieve commercial standards of furfural and glucose production, several process control philosophies are proposed, including installing temperature controllers, pressure controllers, and composition analyzers on the distillation column or its nearby stream (Li et al. 2019). In this context, a composition analyzer was installed at the product outlet streams of the distillation column to ensure the purity of the products (i.e., furfural and glucose) met the commercial standard. Additionally, temperature and pressure controllers are installed on the distillation column to prevent unforeseen incidents, such as oversupply of hot pressurized steam and runaway reaction. Notably, in order to meet sterilization and cleaning standards of the food industry, the outlet temperature of glucose products at the distillate stream must be set above 110 °C to disinfect the glucose products (Błaszczyk and Iciek 2021; Mironescu and Mironescu 2006).
Overall, the total cost of IBL is calculated as USD 10.50 million. The major cost driver of direct cost is the saccharification unit which takes 43.51% (USD 4.57 million) of IBL cost. This is because eight reactors are used for higher conversion into furfural using cellulase enzyme. Other than a reactor, a distillation column has a higher price in purification units.
OBL cost is identified for each facility which is estimated at a percent of FCI (Peters et al. 2003). These general facilities include buildings, yard improvements, communication, sanitary disposal, and safety installation follow similar factors as well. The total cost of OBL was calculated as USD 4.66 million. On the other hand, indirect costs include legal expenses, construction costs, and contractor fees, and the cost estimation is similar to the OBL cost (Peters et al. 2003). Overall, the total indirect cost is calculated as USD 5.65 million.
Operating cost
Operating costs comprise both the manufacturing and non-manufacturing costs of the plant. The manufacturing costs comprise variable and fixed costs, where variable cost includes labor wages, maintenance costs, insurance, and interest, and fixed cost includes raw materials and utility costs incurred annually (Malaysia Indeed 2021; Peters et al. 2003). Non-manufacturing cost includes administration, research, and development, as well as distribution and marketing. The total unit operating cost and annual operating capital are calculated as USD 1138.66/tonne of glucose and USD 16.93 million/year, respectively. In the plant, cellulase enzyme has contributed 85.53% (USD 2.79 million/year) of feedstock cost due to the high cost of cellulase and the absence of an enzyme recovery unit. Nonetheless, employing cellulase improves the conversion of furfural and eventually resulting in higher profits.
Working capital
Working capital is the money needed to start up and run a plant until it generates enough revenue to pay off debt and purchase inventory (Sinnott and Towler 2020). The raw materials and final products stock have three weeks inventory, and water has one week inventory as it is easily available (Sinnott and Towler 2020). It is assumed that six weeks inventory value is allocated to both debtors and creditors. The working capital is calculated as USD 3.74 million with the previous inventories.
Profitability evaluation
Cumulative cash flow
The production plant runs for 20 years in Malaysia, with an additional year for planning and design in 2021, two years for building and installation, and one year for decommissioning in the final year (2044). It is assumed that 70% of the total working capital is used in that year, and 15% of total working capital is shared equally in the following years as the growth rate in production is linear. With a declining balance, the fixed capital begins to depreciate at a rate of 13.91%. In Malaysia, a company that has a fixed capital of above USD 0.61 million pays a 24% corporate tax (LHDN Malaysia 2020; XE currency 2021). The impact of inflation during the lifetime of plant operation is estimated to be negligible. Figure 7 shows the plant recovering full FCI at the initial stage of the 9th year and profiting until the 22nd operating year. The remaining residual value and working capital are anticipated to be recovered at the end of the project.
Net Present Value, Internal Rate of Return, and Return on Investment
The discounted cash flow after tax is used to compute this project’s net present value (NPV). The discount rate is assumed to be 10% as the source of funds between equity and loan is uncertain at this stage of the project. Based on the assumptions, the NPV is calculated as USD 7.65 million by summing discount cash flows after tax at a 10% discount rate throughout 23 years. The discount rate that gives a zero NPV is the internal rate of return (IRR). Figure 8 shows a graph of NPVs against a range of discount rates where the IRR value is calculated to be 14.25%. Moreover, the return on investment (ROI) is determined using the annual operating profit and total investment, which is calculated to be 22.06% (Fig. 9).
Sensitivity analysis
Using the cumulative cash flow table, the various parameters affecting the profitability of the plant can be varied to calculate the change in NPV and IRR. With a positive IRR, the NPV of the plant increases, thus increasing its profit. On the other hand, negative NPV and IRR indicated a decrease in plant profit. This parametric change must be avoided to maintain the profitability of the plant.
The tornado chart shows that capacity is the major cost driver and cutting capacity by 20% dramatically reduces the NPV and IRR. To make a plant profitable, its capacity should be expanded or maintained. Aside from capacity, the selling price of furfural significantly influences NPV and IRR. From the market analysis, the furfural selling price is very elastic and has fluctuated drastically over the past few years (Mordor Intelligence 2021). Furthermore, the fixed operating costs, FCI, and glucose selling price have a lower impact on the profitability of the plant.
Plant viability
Constructing an integrated process plant in Malaysia that uses EFB to manufacture glucose and furfural will result in a profitable process plant. According to market research, the demand for glucose and furfural is increasing rapidly in Malaysia (Persistence Market Research 2020). Therefore, the process plant can be upscaled in the future for a higher profit. Nonetheless, the plant's capacity is not projected to decline, implying that the plant will be profitable in the future. In addition, the selling price of furfural fluctuates greatly, currently showing a lower value in 2021 (Mordor Intelligence 2021). As Malaysia is going through the phase of vaccination for the COVID-19 coronavirus, the economy of all the process plants is affected. After the vaccination phase, the country’s economy is expected to recover, leading to a higher product selling price and higher NPV (Tables 9, 10, 11, and 12).
In 2020, the palm oil sector was the greatest contributor to the Agricommodity crops of Malaysia, contributing USD 11.65 billion, or 3.6% of the total gross domestic product (GDP), and is forecasted to expand to USD 16.75 billion by 2030 (Ministry of Plantation Industries and Commodities 2021; XE currency 2021). However, the potential of downstream activities has not yet been fully developed, resulting in the abundance of biomass produced by the industry. Hence, this proposed integrated plant that produces value-added products (glucose and furfural) in palm oil downstream segments is critical in achieving the GDP target of USD 16.75 billion by 2030 in the palm oil industry.
In terms of operating costs, the cellulase enzyme (catalyst) is the major cost driver of the process plant using EFB, which is cheap biomass available in Malaysia. As the research is conducted through simulation, the flow of catalyst and catalyst recycling is not considered, where installing an enzyme separation and recycling stream can reduce the variable cost greatly. In terms of FCI, the enzymatic reactor has the highest equipment cost because of its large residence time. Optimizing the reactor process condition can reduce the residence time resulting in a smaller reactor. Finally, various parameters can be further optimized in the process plant in the future, allowing for increased profit and sustainability.
Conclusion
In this work, an integrated process plant producing both glucose and furfural from palm oil empty fruit bunches (EFB) is designed and simulated using the most suitable process conditions to maximize the yield and purity of the product. The mass and energy balance results are obtained, and a process flow diagram is drawn to support the equipment used in the plant. For a production capacity of 10 ktpy of glucose and 4.96 ktpy of furfural, purity of 98.21% and 99.54% is achieved, respectively. The plant economy is analyzed by obtaining the fixed capital investment (FCI), operating costs, and working capital, where the profitability is analyzed using cumulative cash flow (CCF), net present value (NPV), and internal return rate (IRR). For the capacity of 10 kilotons per year (ktpy) of glucose and 4.96 ktpy of furfural, the FCI is calculated as USD 20.80 million. The working and operating costs are calculated as USD 3.74 million and USD 16.93 million, respectively. On the evaluation of the profitability of the plant, the NPV, IRR, and return on investment (ROI) of the process plant are calculated as USD 7.65 million, 14.25%, and 22.06%, respectively. From the obtained results, it can be concluded that the designed process plant is profitable, and this profit can be increased with optimization, which is suggested to be included in the future. Therefore, the designed integrated process plant reduces the waste from the palm oil mill, which makes it both a profitable and sustainable plant. Integrated production of furfural and glucose using EFB-hemicellulose biomass is a promising pathway. Considering the residue (i.e., cellulose and lignin) produced from the proposed plant, future work on converting the residue to valuable products is suggested. For instance, hydrogen that acts as an alternative clean energy carrier to non-renewable fossil fuels can be produced from the EFB residue. To the best of our knowledge, no commercial EFB-based hydrogen production plant is currently available. Indeed, it is worthwhile to study hydrogen production from EFB components so that, in the future, not only food and chemicals but also food, chemicals, and energy can be produced from EFB to fully utilize the potential of EFB. Potential synergistic utilization of similar raw materials and utilities in the food, environmental, and energy nexus to produce value-added outputs makes co-location principles economically and commercially attractive.
Data availability
Reasonable data will be made available upon request.
Abbreviations
- MSW:
-
Municipal solid waste
- MT:
-
Metric tons
- EFB:
-
Empty fruit bunch
- MEBT:
-
Mass and energy balance table
- GWP:
-
Global warming potential
- DE:
-
Dextrose equivalent
- CARG:
-
Compound annual growth rate
- ktpy:
-
Kilotons per year
- tpy:
-
Tons per year
- CIP:
-
Cleaning in place
- HACCP:
-
Hazard analysis critical control points
- H2O:
-
Water
- PFD:
-
Process flow diagram
- wt%:
-
Weight percentage
- ppm:
-
Parts per million
- °C:
-
Degree Celsius
- FCI:
-
Fixed capital investment
- NPV:
-
Net present value
- IRR:
-
Internal rate of return
- ROI:
-
Return on investment
- CCF:
-
Cumulative cash flow
- VLE:
-
Vapor liquid equilibrium
- LLE:
-
Liquid–liquid extraction
- CPSPO1-1:
-
Solid molar volume
- VSPOLY-1:
-
Solid heat capacity
- GDP:
-
Gross domestic product
References
21Food (2021) Glucose syrup specification. https://www.21food.com/products/glucose-syrup-2025807.html. Accessed 12 March 2021
Ahmad FB, Zhang Z, Doherty WOS, O’Hara IM (2019) The outlook of the production of advanced fuels and chemicals from integrated oil palm biomass biorefinery. Renew Sust Energ Rev 109:386–411. https://doi.org/10.1016/j.rser.2019.04.009
Alaw FA, Sulaiman, NSa, (2019) A review of boiler operational risks in empty fruit bunch fired biopower plant. J Chem Eng Ind Biotechnol 5:29–35
Aydin G (2015) Performance of recycling abrasives in rock cutting by abrasive water jet. J Cent South Univ 22:1055–1061
Aydin G, Kaya S, Karakurt I (2017) Utilization of solid-cutting waste of granite as an alternative abrasive in abrasive waterjet cutting of marble. J Clean Prod 159:241–247
Aziz M, Oda T, Kashiwagi T (2015) Design and analysis of energy-efficient integrated crude palm oil and palm kernel oil processes. J Jpn Inst Energy 94:143–150
Banach JL, van den Burg SWK, van der Fels-Klerx HJ (2020) Food safety during seaweed cultivation at offshore wind farms: An exploratory study in the North Sea. Mar Policy 120:104082. https://doi.org/10.1016/j.marpol.2020.104082
Basso A, Serban S (2020) Overview of immobilized enzymes’ applications in pharmaceutical, chemical, and food industry. In: Immobilization of enzymes and cells, Springer, pp. 27–63
Błaszczyk I, Iciek J (2021) Selection of heat treatment conditions and prevention of secondary microbial contamination of liquid sugar: practical remarks. J Food Sci Technol 58:2842–2846. https://doi.org/10.1007/s13197-021-04971-3
Buaisha M, Balku S, Özalp-Yaman S (2020) Heavy metal removal investigation in conventional activated sludge systems. Civ Eng J 6:470–477
Celep O, Aydin G, Karakurt I (2013) Diamond recovery from waste sawblades: a preliminary investigation. Proc Inst Mech Eng b: J Eng Manuf 227:917–921
Chaib R, Barone M (2020) The future of chemicals in the food production ambit. In: Chaib R, Barone M (eds) Chemicals in the food industry: toxicological concerns and safe use. Springer International Publishing, Cham, pp 67–70
Chiesa S, Gnansounou E (2014) Use of empty fruit bunches from the oil palm for bioethanol production: a thorough comparison between dilute acid and dilute alkali pretreatment. Bioresour Technol 159:355–364
Chiew YL, Shimada S (2013) Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer – A case study of Malaysia. Biomass Bioenergy 51:109–124. https://doi.org/10.1016/j.biombioe.2013.01.012
Choi J-H, Jang S-K, Kim J-H, Park S-Y, Kim J-C, Jeong H, Kim H-Y, Choi I-G (2019) Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew Energy 130:952–960. https://doi.org/10.1016/j.renene.2018.05.052
Dolphin Centrifuge (2021) Decanter centrifuge. https://dolphincentrifuge.com/difference-between-decanter-centrifuge-disc-centrifuge/. Accessed 6 April 2021
Ebert J (2008) Furfural: future feedstock for fuels and chemicals. In: Biomass magazine
Ellis C (2018) World Bank: global waste generation could increase 70% by 2050. https://www.wastedive.com/news/world-bank-global-waste-generation-2050/533031/. Accessed 2 March 2021
Fisher Scientific (2022) D-(+)-Glucose 98.0+%, TCI America. https://www.fishersci.com/shop/products/d-glucose-tci-america-2/G004825G. Accessed 19 May 2022
Geng A (2013) Conversion of oil palm empty fruit bunch to biofuels. Liquid, gaseous and solid biofuels-conversion techniques, InTech, Croatia, pp 479–490.
Hafyan RH, Bhullar LK, Mahadzir S, Bilad MR, Nordin NAH, Wirzal MDH, Putra ZA, Rangaiah GP, Abdullah B (2020) Integrated biorefinery of empty fruit bunch from palm oil industries to produce valuable biochemicals. Processes 8:868
Hongye Holding Group Corporation Ltd. (2020) Furfural specification. https://www.go4worldbusiness.com/product/view/1143815/furfural.html. Accessed 15 March 2021
Humbird D, Davis R, Tao L, Kinchin C, Hsu D, Aden A, Schoen P, Lukas J, Olthof B, Worley M (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover. In: National Renewable Energy Lab.(NREL), Golden, CO (United States).
Malaysia Indeed (2021) Average base salary in Malaysia 2021. https://malaysia.indeed.com/career/factory-worker/salaries. Accessed 30 April 2021
Mordor Intelligence (2021) Furfural market – growth, trends, covid-19 impact, and forecast (2021–2026). https://www.mordorintelligence.com/industry-reports/furfural-market. Accessed 13 March 2021
IHS Markit (2020) Furfural -chemical economics handbook. https://ihsmarkit.com/products/furfural-chemical-economics-handbook.html. Accessed 15 March 2021
Jin Q, Yang L, Poe N, Huang H (2018) Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci Technol 74:119–131
Jung YH, Kim IJ, Kim HK, Kim KH (2013) Dilute acid pretreatment of lignocellulose for whole slurry ethanol fermentation. Bioresour Technol 132:109–114. https://doi.org/10.1016/j.biortech.2012.12.151
Kenthorai Raman J, Gnansounou E (2015) Furfural production from empty fruit bunch – A biorefinery approach. Ind Crops Prod 69:371–377. https://doi.org/10.1016/j.indcrop.2015.02.063
Li J, Zhou H, Sun L, Zhang N (2019) Design and control of different pressure thermally coupled reactive distillation for synthesis of isoamyl acetate. Chem. Eng. Process. Process Intensif. 139:51–67. https://doi.org/10.1016/j.cep.2019.03.014
Lightfoot NF, Maier EA (1998) Chapter 6 - materials. In: Lightfoot NF, Maier EA (eds) Microbiological analysis of food and water. Elsevier Science B. V, Amsterdam, pp 87–108
Liquiflo (2016) Liquiflo chemical processing pump. http://www.liquiflo.com/v2/files/pdf/applicationnotes/AN1602-4-MinFlowRateforCentrifugalPumps-Feb2016.pdf. Accessed 5 April 2021
Liu Z, Shi E, Ma F, Jiang K (2021) An integrated biorefinery process for co-production of xylose and glucose using maleic acid as efficient catalyst. Bioresour Technol 325:124698. https://doi.org/10.1016/j.biortech.2021.124698
Loginova I, Kharina M, Kanarskaya Z, Meshcheryakova M, Dubkova N (2021) Complex processing of plant raw materials for furfural and glucose production. In: IOP conference series: earth and environmental science, Vol. 677, p 052014, IOP Publishing.
Loh SK (2018) Optimisation of process conditions for ethanol production from enzymatically saccharified empty fruit bunch using response surface methodology (RSM). J Oil Palm Res. https://doi.org/10.21894/jopr.2018.0045
LHDN Malaysia (2020) Corporate tax rate in 2020. http://www.hasil.gov.my/bt_goindex.php?bt_kump=5&bt_skum=2&bt_posi=5&bt_unit=1&bt_sequ=1. Accessed 30 April 2021
Malliaroudaki MI, Watson NJ, Ferrari R, Nchari LN, Gomes RL (2022) Energy management for a net zero dairy supply chain under climate change. Trends Food Sci Technol
Manual AP (2001) Physical property systems, physical property methods and models 11.1. Aspen Technology Inc.
Ministry of Plantation Industries and Commodities (2021) National Agricommodity Policy 2021- 2030 (DAKN2030), Ministry of Plantation Industries and Commodities (MPIC), Malaysia.
Mironescu M, Mironescu V (2006) Hygienic aspects at the production of glucose syrups through acid hydrolysis. Acta Univ Cibiniensis Ser e Food Technol 10:29
Mittal A, Black SK, Vinzant TB, O’Brien M, Tucker MP, Johnson DK (2017) Production of furfural from process-relevant biomass-derived pentoses in a biphasic reaction system. ACS Sustain Chem Eng 5:5694–5701
Moerman F, Rizoulières P, Majoor FA (2014) 10 - Cleaning in place (CIP) in food processing. In: Lelieveld HLM, Holah JT, Napper D (eds) Hygiene in food processing, 2nd edn. Woodhead Publishing, pp 305–383
Montané D, Salvadó J, Torras C, Farriol X (2002) High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenergy 22:295–304. https://doi.org/10.1016/S0961-9534(02)00007-7
Motarjemi Y, Lelieveld H (2013) Food safety management: a practical guide for the food industry. Academic Press
MPCO (2021) Malaysian Palm Oil Industry. http://mpoc.org.my/malaysian-palm-oil-industry/. Accessed 4 March 2021
Nhien LC, Long NVD, Kim S, Lee M (2017) Techno-economic assessment of hybrid extraction and distillation processes for furfural production from lignocellulosic biomass. Biotechnol Biofuels 10:81–81. https://doi.org/10.1186/s13068-017-0767-3
Nhien LC, Long NVD, Lee M (2021) Novel hybrid reactive distillation with extraction and distillation processes for furfural production from an actual xylose solution. Energies 14:1152
Ninduangdee P, Kuprianov VI (2016) A study on combustion of oil palm empty fruit bunch in a fluidized bed using alternative bed materials: Performance, emissions, and time-domain changes in the bed condition. Appl Energy 176:34–48
Orugba HO, Oghenejoboh KM, Oghenejoboh UM, Ohimor OE (2021) Production of biodiesel from a novel combination of raphia africana kernel oil and turtle shell (Centrochelys Sulcata) Heterogenous Catalyst. J Hum Earth, Future 2:258–268
Persistence Market Research (2020) Glucose and fructose market. https://www.persistencemarketresearch.com/market-research/glucose-and-fructose-market.asp. Accessed 12 March 2021
Peters MS, Timmerhaus KD, West RE (2003) Plant design and economics for chemical engineers. McGraw-Hill, New York
Pina EA, Palacios-Bereche R, Chavez-Rodrigues MF, Ensinas AV, Modesto M, Nebra SA (2014) Thermal integration of different plant configurations of sugar and ethanol production from sugarcane. Chem Eng 39
Sarungalo Z (2005) Production and characterization of glucose syrup of papuan sago starch. In: Sago palm development and utilization. Proceeding of the eight international sago symposium (8ISS), Universitas Negeri Papua, Indonesia
Sheppard P, Garcia-Garcia G, Angelis-Dimakis A, Campbell GM, Rahimifard S (2019) Synergies in the co-location of food manufacturing and biorefining. Food Bioprod Process 117:340–359
Sigma-Aldrich (2022) Furfural. https://www.sigmaaldrich.com/MY/en/product/aldrich/w248908. Accessed 19 May 2022
Sinnott R, Towler G (2020) Chapter 6—costing and project evaluation. In: Sinnott R, Towler G (eds) Chemical engineering design (Sixth Edition). Butterworth-Heinemann, pp 275–369
Solomou K, Alyassin M, Angelis-Dimakis A, Campbell GM (2022) Arabinoxylans: A new class of food ingredients arising from synergies with biorefining, and illustrating the nature of biorefinery engineering. Food Bioprod Process 132:83–98. https://doi.org/10.1016/j.fbp.2021.12.007
The World Bank (2021) Trends in solid waste management. https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html. Accessed 30 December 2021
Umana US, Ebong MS, Godwin EO (2020) Biomass production from oil palm and its value chain. J. Hum Earth, Future 1:30–38
UN Data (2018) Glucose and furfural Data. http://data.un.org/. Accessed 12 March 2021
Wilson R, Lilly M (1969) Preparation and use of insolubilized amyloglucosidase for the production of sweet glucose liquors. Biotechnol Bioeng 11:349–362
Wooley RJ, Putsche V (1996) Development of an ASPEN PLUS physical property database for biofuels components. United States.
XE currency (2021) Conversion rate of USD to MYR in May 2021. https://www.xe.com/currencyconverter/convert/?Amount=1&From=USD&To=MYR. Accessed 30 April 2021
Zafar S (2021) Everything you need to know about biomass energy system. https://www.bioenergyconsult.com/tag/biomass-wastes/. Accessed 5 March 2021
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Competing interests
The authors do not have any conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammed, M.Z.R., Ng, Z.W., Putranto, A. et al. Process design, simulation, and techno-economic analysis of integrated production of furfural and glucose derived from palm oil empty fruit bunches. Clean Techn Environ Policy 25, 1551–1567 (2023). https://doi.org/10.1007/s10098-022-02454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-022-02454-3