Abstract
The increasing generation of plastic wastes forces us to search for final disposal technologies environmentally friendly such as pyrolysis, which becomes an interesting technique because it takes advantage of the wastes obtaining important products. In addition, catalytic pyrolysis by using commercial catalysts, e.g. such zeolites, alumina or recovered from other industrial processes, it allows decreases the activation energy and selectivity in the obtained products. In this study, we report the evaluation of the catalytic pyrolysis with a regenerated fluid catalytic cracking catalyst using thermogravimetry with polypropylene and a pyrolytic process carried out in a batch reactor with polypropylene in a 1:10 ratio (catalyst-plastic). The regeneration studies were carried using two solvents (ethanol and toluene) at different contact times, then a thermal regeneration at two heating ramps was performed and the best treatment was evaluated by scanning electron microscopy energy-dispersive X-ray spectroscopy and surface area analysis. The results showed a better action of the ethanol in the chemical treatment at 14 h of contact in the heat treatment due to longer gasification of the coke. The degradation process using recovered catalyst decreases the degradation temperature compared to the no-catalyst process. As a consequence, the yield of the liquid fraction decreases by 10% with greater orientation to aliphatic components.
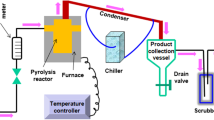
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, there are serious problems of environmental pollution due to the considerable increase in the generation of plastic waste, high production of waste catalysts from oil refineries and high CO2 emissions from fossil sources such as gas, coal and oil (Palmay et al. 2022). Promoting the use of new sustainable and efficient alternatives through reduction, recycling and reuse activities allow to solve these problems mentioned above (Miandad et al. 2019). In this context, gasification, thermal and catalytic pyrolysis are technologies with various operational and environmental advantages for energy recovery, which are becoming alternative methods for clean energy production, especially in processes involving plastic waste. The pyrolysis process allows anaerobic thermochemical decomposition at high temperature of plastic waste through an inert nitrogen atmosphere, which removes the oxygen from the molecules and consequently, it is possible to convert the plastic into liquid oils (short-chain hydrocarbons) similarly to conventional fuels (gasoline and diesel), solids (carbon) and gases (hydrocarbon isomers between C1 and C5). In addition, other factors such as the heating rate, the type of plastic, the type of reactor and the presence of catalysts influence the products from pyrolysis quality (Anuar Sharuddin et al. 2016).
Different catalysts have been used in the catalytic degradation of plastic waste such as amorphous silica-aluminas, acid catalysts, ZSM-5, red mud, HY zeolites (Heracleous et al. 2019; Rehan et al. 2017), which accelerates the process and favours the quality of the pyrolytic products, obtaining high selectivity and a liquid fraction mainly in diesel-like compounds, where fractions distribution of the catalytic pyrolytic products are influenced mainly by the catalyst used. A great disadvantage of the use of these catalysts is their economic cost, however, the recycling of catalysts from fluid catalytic cracking (FCC) units together with plastic waste has shown great potential in catalytic pyrolysis, being a more profitable alternative compared to other catalysts. In this way, the FCC catalytic pyrolysis of polyolefins such as polyethylene (PE) and PP studied have shown a yield of 92.3% for the highest liquid fraction at 450 °C (Abbas-Abadi et al. 2014). Aisien et al. (2021) reported a liquid fraction yield of 83.3% using commercial FCC catalysts in PP pyrolysis at different temperatures and a yield of 77.6% using FCC spent catalyst at 450 °C. The influence of the catalysts on the obtained fractions depends on the porous surface of the catalyst and its acidity, which can give rise to uncontrolled fractionation, reducing the liquid fraction and increasing the gases produced when its acidity is excessively high (Lin and Yang 2008). Other researchers have studied the catalytic pyrolysis in a mixture of plastics using FCC and ZSM-5 type zeolites with similar conclusions regarding the orientation of the products, however, there was a decrease of 10% in the liquid fraction (Onwudili et al. 2019). In addition, catalysts help to produce lighter liquid fractions with proper characteristics to use in engines, furthermore decreasing the total energy consumed (Miandad et al. 2016).
Despite the advantages presented by the regeneration methods of catalysts from oil refining process, they are limited and still very little exploited. Ding et al., studied the solvent method to remove sulphur of FFC catalysts using carbon disulphide, ethanol and benzene as solvents (Ding et al. 2019), the results showed better yield with carbon disulphide followed by ethanol. Another method to remove sulphur and carbon is by the gasification of catalysts at 450 °C (Su et al. 2019), where they were satisfactory converted into sulphur dioxide and carbon dioxide. The leaching process is another interesting method for this purpose, where lanthanum can be recovered by applying an acid leaching followed by a heat treatment at 750 °C (Zhao et al. 2017).
In this work, a regenerated FCC catalyst from a petrochemical industry applied to a catalytic degradation of polypropylene has been investigated. A chemical regeneration of the catalyst with two solvents at different contact times and a thermal regeneration by gasification using two heating ramps have been performed. This catalyst has been characterized (e.g. surface area) and its behaviour was compared with a commercial catalyst by means thermogravimetry. Therefore, it was used to evaluate the influence in the polypropylene (PP) pyrolysis process and to establish its relationship with the quality of the catalytic products obtained, their yields and economic costs. In addition, it is a great alternative to close the circle of the circular economy by reusing both recycled plastic and spent FCC catalyst from refineries (hazardous waste due to the presence of heavy metals in its composition) (Wang et al. 2021a, b).
Materials and methods
Physicochemical characterization of polypropylene plastic waste
PP has been characterized by Fourier Transform Infrared Spectroscopy (FTIR) using a JASCO FT/IR-4100 spectrometer. This method used was executed with the Spectra Analysis program, which performs the data acquisition and treatment, and provides a numerical value based on the height or area of the peak in a working scan range of 4000–400 cm−1 (Palmay et al. 2021a, b).
Spent catalyst conditioning
One of the most important processes in oil refining is fluidized catalytic cracking (FCC), in this process, the catalysts become saturated and/or poisoned by the deposition of coke or metallic elements, for this reason, the catalysts are thrown out periodically (Almas et al. 2019). In the present study, 20 kg of spent catalyst based on zeolites have been sampled randomly from a standard production day in an oil refining industry. The catalyst was heated at 140° C for 2 h to remove the absorbed water during final storage.
Regeneration of spent catalyst
The regeneration takes place in two stages. In the first one, chemical treatment has been carried out to remove sulphides or sulphur oxides adsorbed into the catalyst structure during the FCC unit operation. Solutions of 200 mL of each solvent (ethanol or toluene) were stirred at 300 rpm between 12 and 14 h with the spent catalyst, using 10 mL of solvent per gram of catalyst. The resulting solution have been filtered and the solid product was dried at 120 °C for 2 h for complete evaporation of the solvent. The sample produced in chemical treatment, in the second stage was calcinated at three heating temperatures, the first one at 350 °C for 1 h at 50 °C h−1 of heating rate, then at 450°C for 1,5 h at 25 °C min−1 of heating rate, and the last one at 700 °C for 2 h at 50 °C h−1 of heating rate. Finally, the samples were cooled gradually, we can see in Fig. 1 the behaviour of the heating.
Characterization of the regenerated catalyst
Porosity and surface area analysis (BET method)
The surface area of the catalyst was carried out with the BET method using Micromeritics AutoChem 2920 equipment, (series 413,841/14) in a dynamic flow mode with nitrogen. The container of 500 mL of N2 and the equipment ramp were configured to perform the adsorption and desorption cycles at the nitrogen liquefaction (− 195.6 °C). The surface area of the samples and also a porosity size distribution was determined by calculating the number of adsorbate molecules.
X-ray diffraction analysis (XRD)
X-ray diffraction (XRD) patterns of the regenerated catalyst were performed to determine its crystal structure. There are some types of zeolites, each one has a specific crystalline characteristic and spatial arrangement, which allows identifying the type and purity of zeolite that has been used (Kanchanatip et al. 2022).
Scanning electron microscopy analysis (SEM–EDS)
SEM–EDS provides important information for the study of catalysts because depending on the energy of the electrons bombarded by the SEM's electron beam, other electrons are ejected from the atoms comprising the sample's surface or the internal structure of the solid (Akubo et al. 2019). SEM images were obtained with a TESLA instrument in connection with a TESCAN digitizing unit. The X-ray energy is characteristic of the element from which it was emitted and provides information on the chemical composition. The samples were calcined before the analysis in SEM–EDS (JSM-IT100LA). Samples were fixed on Stubs for SEM using conductive double-sided carbon tape and then covered with conductive gold (99.99%) for 30 s.
Thermogravimetric analysis of polypropylene using the regenerated catalyst
Thermogravimetric analysis (TGA) is used to perform the characterization of physicochemical properties of some materials, mass variation is measured while the sample is heated in a defined atmosphere and controlled conditions (Wang et al. 2021a, b). In this experiment, recycled, washed and crushed polypropylene samples were placed into TGA 1 STAR System equipment (METTLER TOLEDO, precision of ± 0.001 mg) with nitrogen atmosphere at 20 mL min−1 flow. The tests were carried out in a dynamic state from room temperature to 550 °C at a 15 °C min−1 heating rate. We developed a comparison of the catalytic activity in three variables: No Catalyst (SC), Commercial Catalyst (CC) and Regenerated Catalyst (CR), in 1:10 ratio catalyst—plastic fed in all the experiments. In each case, the maximum degradation temperature was determined by the first derivative of the mass variation against temperature, in addition, the maximum conversion in each treatment was calculated.
Polypropylene catalytic pyrolysis process
The evaluation of the catalyst in the catalytic pyrolysis has been developed in a batch reactor at the operating conditions that are shown in Table 2. The liquid fraction produced was condensed at 10 °C, then collected in amber bottles and preserved at the same temperature. The gaseous fraction was calculated by a balance of materials in the reactor. The experiments were carried out by triplicate using 200 g of crushed-recycled PP. (Palmay et al. 2021a) In addition to the gaseous and liquid products, there was also a solid deposit (coke) formed on the catalyst causing its deactivation.
Results and discussion
Characterization of recycled polypropylene (PP)
FTIR has been used for the chemical characterization of plastic waste as shown in Fig. 2. In the PP spectrum, three groups of bands can be seen corresponding to tension movements of the C–H bonds at 2900 cm−1, C–C tension movements at 1350–1470 cm−1 and a bending movement of –CH3 between 1200 and 1000 cm−1. In addition, these results are similar to those reported in another work carried out by the authors (Palmay et al. 2021a, b), which shows that the biomass used in this study to carry out the FCC catalytic pyrolysis was polypropylene.
Characterization of the regenerated catalyst
Table 1 presents the BET pore sizes and specific surface areas, pore volumes of regenerated catalyst. As we can see, based on the surface area, ramp 2 shows better results, furthermore, ethanol has the highest solvent action to remove pollutants from the catalyst. Similarly, in 12 h of contact time in the mixture (solvents and catalyst) in ramp 2 give rise to an increase in pore size of the catalyst that could be attributed to the complete remotion of coke in gasification at higher temperature in the regeneration process (Moorthy Rajendran et al. 2020). On the other hand, toluene in ramp 2 does not present the same behaviour, since the sintering process occurs with molecules of the solid that form disordered and conglomerated structures around a point (Germania et al. 2018). To determine the efficiency, the catalyst with the highest surface area was selected and used in thermal degradation tests by dynamic thermogravimetric analysis at a heating rate of 15 °C min−1 as shown in Table 3.
In previous studies of the FCC catalyst analysis (Carrera 2013), the BET surface area of a new catalyst of 250 m2 kg−1, which after compared to that obtained after regeneration, presents in all cases a decrease of more than 50%, which can be attributed to the treatments as well as to the metal poisoning or the regeneration temperature of 700 °C applied, which causes the crystalline structure of the zeolites to be modified to improve its stability, generating a change in the porosity. Additionally, a low surface area is accompanied by a high porosity which is attributed to the mechanical wear suffered by the material during the gas flow in the FCC operation, however, this decrease in surface area is compensated by its low cost compared to synthetic catalysts used for these operations.
The influence of the catalyst in the degradation reaction generates a slight decrease in the maximum degradation temperature compared to the thermal process presented in previous studies where maximum temperatures were 450 °C (Lin and Yang 2008; Vogt et al. 2015).
Figure 3 shows the XRD patterns of the regenerated FCC catalyst using a Malvern Panalytical Empyrean instrument with Cu radiation. The tests were conducted with a step size of 0.020°, for 2θ values between 5 and 90°. The characteristic peaks of zeolite Y could be observed at 2θ values of 10, 12 16, 19 21, 24, 27 and 32.0° (Salahudeen 2019). Other background peaks present were due to the support materials such as faujasite (active matrix together with the zeolite Y) and montmorillonite (inert matrix of the catalyst). In addition, through diffractogram analysis, a crystallinity of 71.3% zeolite Y, 2.2% faujasite and 26.5% montmorillonite was detected.
In Table 4, results of the energy-dispersive X-ray spectroscopy (EDS) analysis of the regenerated catalyst in Ramp 1 at 20.5 h and Ramp 2 at 22.5 h are expressed as a percentage in mass. High coke removal is achieved; therefore, an increase would be expected in aluminium and silicon concentration in the samples after a mass balance is performed in the absence of the contaminants. To evaluate catalyst regeneration, the results of this investigation have been compared with those of an EDS analysis of the spent catalyst without chemical treatment in the previous investigation developed in the same conditions. The results show Al: 20.55%, Si: 23.56%, Fe: 0.66% and La: 3.08% percentage in mass (Wang et al. 2021a, b).
The aggressiveness of the regeneration chemical treatment is evidenced through the loss of the sample (Al and Si loss). The EDS results show the great solvency power of toluene with the conditions in ramp 1 of the heat treatment in 14 h. For the other metals (Fe and La), the concentration is not influenced significantly. This behaviour is due to the used solvent in the regeneration treatment to remove coke and sulphur that have no affinity towards Fe and La metallic phase since these metals in normal conditions are soluble in strong acids. One of the characteristics of catalysts is their acidity, which depends on Si/Al ratio and it allows the evaluation of their catalytic activity. However, lanthanum can be impregnated in the catalyst support to improve its operating properties (resistance to wear and operating temperature), consequently modifying the acidity but this modification helps in the breakdown of high molecular weight molecules (Su et al. 2019; Zhou et al. 2020).
We can see in Table 4 that Si/Al ratios are close to 1, which is characteristic of spent catalysts. So we could assume that despite removing pollutants (sulphur and coke) from the catalyst, the concentration of the main elements has not changed significantly. The degree of regeneration of the catalyst has been evaluated by comparing the recovered catalyst Si/Al ratio to the results of Si/Al ratio for new catalysts, reported by Kassargy et al. (2017).
A simulation of the catalytic pyrolysis process is shown in Fig. 4. First, organic radicals are generated by decomposition reactions as a result of heat, then the catalyst allows the reaction of the radicals coming from the degraded plastic, reactions that are developed at specific points (active side) on the surface and finally promote them to form the products (Aljabri et al. 2017; Arandes et al. 1997; Lopez et al. 2017).
2 mg of regenerated catalyst were adhered to a carbon fibre tape and analysed under vacuum at 1 Pa by SEM. Under these conditions, SEM images were obtained as shown in Fig. 5. Figure 5a corresponds to the micrograph of the solid samples after the calcination using ramp 1 and pretreated with ethanol for 12 h, while Fig. 5b belongs to samples calcinated using ramp 2 and pretreated with ethanol for 12 h. The gasification of pollutants and porosity formation is an effect of temperature that we can see on the surface of the catalyst. The average diameter of particles in Fig. 5a is 42 µm and 17 µm for particles in Fig. 5b, where the thermal process treatment was much more aggressive increase in cracks by approximately 57%. As a consequence, the catalyst has high porosity that generates greater surface area increasing the contact area between the catalyst surface and the hydrocarbon macromolecules.
Thermogravimetric analysis of PP with catalyst
The regenerated catalyst heated in ramp 2 using ethanol in 14 h had the best properties after the thermal and chemical treatment and have been chosen according to the greater surface area, as well as, the selected solvent generates less environmental impact. The catalyst and polypropylene were studied with thermogravimetric analysis (TGA), to determine the catalytic pyrolysis kinetics. According to the results (Table 5) of the thermogravimetric analysis of PP with catalyst, the presence of catalyst increases the loss mass of the degraded plastic (30%), besides contributing to decreasing the degradation temperature. In addition, there are no significant differences between the new catalyst and the recovered one neither in the percentage of the lost mass nor the maximum degradation temperature. Based on these results we can conclude the regeneration is efficient enough for its application in processes of this type. It is important to mention that the regeneration of catalysts helps to reduce the costs of final disposal of these wastes since the recovered metals (Pd, Pt, Rh) can be again profitably reused in analytical equipment. The average price of catalyst regeneration for one gram in the proposed treatment is $ 2.20, while one gram of a commercial zeolite Y is $ 25.
Figure 6 shows the TGA and DTG of the three experiments developed without catalyst (SC), using a commercial catalyst (CC) and recovered catalyst (CR) each one in separated experiments. The tendency of the degradation curve and temperature using catalysts evidence that in low temperature the gas production is greater so that is why the solid fraction has been reduced (Kasar et al. 2020); Miandad et al. 2017). In the other hand, the use of catalyst in the reaction decreases la temperature de degradation versus the process without catalyst. The behaviour of the regenerated catalyst and the fresh catalyst was similar, almost 400 °C both presented the change in the slope of the curve and almost 450 °C achieve maximum mass lose. In addition, the results obtained in TGA give a guideline to use in future experiments the applied conditions in this test (batch reactor).
Polypropylene catalytic pyrolysis
To prove the results showed in TGA and to demonstrate the feasibility of using the regenerated catalyst in the recycled PP pyrolysis process, we carried out the experiments in the same conditions. The use of this regenerated catalyst mixing directly with the plastic results in a greater production of the gaseous fraction due to the occurrence of lower molecular mass molecules, as well as, the reduction in the solid and liquid fractions. The presence of a catalyst in the reaction cause the liquid fraction (C6–C20) to decrease (10%), and consequently the gaseous fraction (C1–C5) increase (20%), both in regenerated catalyst and in the commercial one, since there is just 2% of reduction in the liquid fraction due to catalytic effect between the commercial catalyst and the recovered one (Miandad et al. 2017). This behaviour is assumed to be due to the influence of the catalyst when the radicals are produced in the initiation and propagation process of the reaction (Kasar et al. 2020; Lovás et al. 2017).
In the pyrolysis developed without catalyst, the GC analysis shows that the saturated and unsaturated aliphatic components are the main products and less than 1% of the whole products are aromatic compounds (Fig. 7b). In catalytic pyrolysis using regenerated catalyst approximately 34% of the main products are unsaturated aliphatic compounds but the paraffin-based products decrease. The presence of the regenerated catalyst increases the olefins fraction and the aromatic compounds, as a result, these components contribute to increasing the properties such as the octane index in fuels (Aboulkas et al. 2010; Singh et al. 2019). Applying the thermal process, oligomers are the major components in products, whereas in the catalytic process the oligomers are partly hydrogenated generating as consequence products that could be used as fuels (Lewandowski et al. 2019; Moorthy Rajendran et al. 2020).
Conclusions
In this work, an FCC catalyst was regenerated applying chemical and thermal treatment at different conditions and then catalytic pyrolysis of polypropylene has been carried out in a batch reactor using this catalyst. The chemical treatment using ethanol to remove sulphur and coke combined to the thermal process generates a surface area of 112.56 m2 gr−1 in the catalyst applying the conditions in ramp 2 in 14 h.
The TGA proved a decrease in the degradation temperature of the polypropylene using the regenerated catalyst to 443.5 °C in front of the average degradation temperature (467 °C) required in non-catalytic pyrolysis. The catalytic pyrolysis using the regenerated catalyst decreases the liquid fraction (10%) and increases the gaseous fraction (20%) although, guiding the formation of unsaturated aliphatic compounds.
In short, the advantages that we have obtained are the reduction of the required energy for polymer degradation, reuse of the spent catalysts, use of ecological solvents, and above all, the low costs ($ 2.50) per gram of the regenerated catalyst considering the price of a new one.
Data availability
Enquiries about data availability should be directed to the authors.
References
Abbas-Abadi MS, Haghighi MN, Yeganeh H, McDonald AG (2014) Evaluation of pyrolysis process parameters on polypropylene degradation products. J Anal Appl Pyrol 109:272–277. https://doi.org/10.1016/j.jaap.2014.05.023
Aboulkas A, El Harfi K, El Bouadili A (2010) Thermal degradation behaviors of polyethylene and polypropylene. Part I: pyrolysis kinetics and mechanisms. Energy Convers Manag 51(7):1363–1369. https://doi.org/10.1016/j.enconman.2009.12.017
Aisien ET, Otuya IC, Aisien FA (2021) Thermal and catalytic pyrolysis of waste polypropylene plastic using spent FCC catalyst. Environ Technol Innov 22:101455. https://doi.org/10.1016/j.eti.2021.101455
Akubo K, Nahil MA, Williams PT (2019) Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J Energy Inst 92(1):195–202. https://doi.org/10.1016/j.joei.2017.10.009
Aljabri NM, Lai Z, Hadjichristidis N, Huang KW (2017) Renewable aromatics from the degradation of polystyrene under mild conditions. J Saudi Chem Soc 21(8):983–989. https://doi.org/10.1016/j.jscs.2017.05.005
Almas Q, Naeem MA, Baldanza MAS, Solomon J, Kenvin JC, Müller CR, Teixeira Da Silva V, Jones CW, Sievers C (2019) Transformations of FCC catalysts and carbonaceous deposits during repeated reaction-regeneration cycles. Catal Sci Technol 9(24):6977–6992. https://doi.org/10.1039/c9cy01680e
Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manage 115:308–326. https://doi.org/10.1016/j.enconman.2016.02.037
Arandes JM, Abajo I, López-Valerio D, Fernández I, Azkoiti MJ, Olazar M, Bilbao J (1997) Transformation of several plastic wastes into fuels by catalytic cracking. Ind Eng Chem Res 36(11):4523–4529. https://doi.org/10.1021/ie970096e
Carrera H (2013) Evaluación y caracterización del catalizador del proceso de craqueo catalítico fluidizado (FCC) (Vol. 16, Issue 22). Universidad Central del Ecuador
Ding K, Liu S, Huang Y, Liu S, Zhou N, Peng P, Wang Y, Chen P, Ruan R (2019) Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers Manage 196(July):1316–1325. https://doi.org/10.1016/j.enconman.2019.07.001
Germania T, Caterine D, José CM, Bolívar A, Vladimir O (2018) Recovery of heavy metals from the spent catalyst of the hydrotreating unit (HDT) for the use of the impregnation of supported catalysts. Key Eng Mater 792:133–139. https://doi.org/10.4028/www.scientific.net/KEM.792.133
Heracleous E, Pachatouridou E, Hernández-Giménez AM, Hernando H, Fakin T, Paioni AL, Baldus M, Serrano DP, Bruijnincx PCA, Weckhuysen BM, Lappas AA (2019) Characterization of deactivated and regenerated zeolite ZSM-5-based catalyst extrudates used in catalytic pyrolysis of biomass. J Catal 380:108–122. https://doi.org/10.1016/j.jcat.2019.10.019
Kanchanatip E, Chansiriwat W, Palalerd S, Khunphonoi R, Kumsaen T, Wantala K (2022) Light biofuel production from waste cooking oil via pyrolytic catalysis cracking over modified Thai dolomite catalysts. Carbon Resour Convers 5(3):177–184. https://doi.org/10.1016/j.crcon.2022.05.001
Kasar P, Sharma DK, Ahmaruzzaman M (2020) Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J Clean Prod 265:121639. https://doi.org/10.1016/j.jclepro.2020.121639
Kassargy C, Awad S, Burnens G, Kahine K, Tazerout M (2017) Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J Anal Appl Pyrol 127(January):31–37. https://doi.org/10.1016/j.jaap.2017.09.005
Lewandowski WM, Januszewicz K, Kosakowski W (2019) Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J Anal Appl Pyrol 140:25–53. https://doi.org/10.1016/j.jaap.2019.03.018
Lin YH, Yang MH (2008) Chemical catalysed recycling of polypropylene over a spent FCC catalyst and various commercial cracking catalysts using TGA. Thermochim Acta 470(1–2):52–59. https://doi.org/10.1016/j.tca.2008.01.015
Lopez G, Artetxe M, Amutio M, Bilbao J, Olazar M (2017) Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew Sustain Energy Rev 73:346–368. https://doi.org/10.1016/j.rser.2017.01.142
Lovás P, Hudec P, Jambor B, Hájeková E, Horňáček M (2017) Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 203:244–252. https://doi.org/10.1016/j.fuel.2017.04.128
Miandad R, Barakat MA, Aburiazaiza AS, Rehan M, Nizami AS (2016) Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot 102:822–838. https://doi.org/10.1016/j.psep.2016.06.022
Miandad R, Barakat MA, Rehan M, Aburiazaiza AS, Ismail IMI, Nizami AS (2017) Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manage 69:66–78. https://doi.org/10.1016/j.wasman.2017.08.032
Miandad R, Rehan M, Barakat MA, Aburiazaiza AS, Khan H, Ismail IMI, Dhavamani J, Gardy J, Hassanpour A, Nizami AS (2019) Catalytic pyrolysis of plastic waste: moving toward pyrolysis based biorefineries. Front Energy Res 7:27. https://doi.org/10.3389/FENRG.2019.00027/BIBTEX
Moorthy Rajendran K, Chintala V, Sharma A, Pal S, Pandey JK, Ghodke P (2020) Review of catalyst materials in achieving the liquid hydrocarbon fuels from municipal mixed plastic waste (MMPW). Mater Today Commun 24(February):100982. https://doi.org/10.1016/j.mtcomm.2020.100982
Onwudili JA, Muhammad C, Williams PT (2019) Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. J Energy Inst 92(5):1337–1347. https://doi.org/10.1016/j.joei.2018.10.001
Palmay P, Morocho S, Donoso C, Puente C (2021a) Thermic pyrolysis of polypropylene waste as a source of fuel. Rev Mex Ing Quím 20(2):1019–1027
Palmay P, Puente C, Barzallo D, Bruno JC (2021) Determination of the thermodynamic parameters of the pyrolysis process of post-consumption thermoplastics by non-isothermal thermogravimetric analysis. Polymers 13(24):4379. https://doi.org/10.3390/polym13244379
Palmay P, Haro C, Huacho I, Barzallo D, Bruno JC (2022) Production and analysis of the physicochemical properties of the pyrolytic oil obtained from pyrolysis of different thermoplastics and plastic mixtures. Molecules 27(10):3287. https://doi.org/10.3390/molecules27103287
Rehan M, Miandad R, Barakat MA, Ismail IMI, Almeelbi T, Gardy J, Hassanpour A, Khan MZ, Demirbas A, Nizami AS (2017) Effect of zeolite catalysts on pyrolysis liquid oil. Int Biodeterior Biodegrad 119:162–175. https://doi.org/10.1016/j.ibiod.2016.11.015
Salahudeen N (2019) Effect of ZSM-5 in the catalytic activity of a fluid catalytic cracking catalyst. J Incl Phenom Macrocycl Chem 93(3–4):173–181. https://doi.org/10.1007/s10847-018-0865-2
Singh RK, Ruj B, Sadhukhan AK, Gupta P (2019) Thermal degradation of waste plastics under non-sweeping atmosphere: Part 1: effect of temperature, product optimization, and degradation mechanism. J Environ Manage 239(March):395–406. https://doi.org/10.1016/j.jenvman.2019.03.067
Su J, Fang C, Yang M, You C, Lin Q, Zhou X, Li H (2019) Catalytic pyrolysis of waste packaging polyethylene using AlCl3–NaCl eutectic salt as catalyst. J Anal Appl Pyrol 139(February):274–281. https://doi.org/10.1016/j.jaap.2019.02.015
Vogt ETC, Whiting GT, Dutta Chowdhury A, Weckhuysen BM (2015) Zeolites and zeotypes for oil and gas conversion. In Advances in catalysis (1st ed., Vol. 58). Elsevier Inc. https://doi.org/10.1016/bs.acat.2015.10.001
Wang Q, Li Y, Benally C, Li Y, Chen C, An Z, Gamal El-Din M (2021a) Spent fluid catalytic cracking (FCC) catalyst enhances pyrolysis of refinery waste activated sludge. J Clean Prod 295:126382. https://doi.org/10.1016/j.jclepro.2021.126382
Wang S, Kim H, Lee D, Lee YR, Won Y, Hwang BW, Nam H, Ryu HJ, Lee KH (2021b) Drop-in fuel production with plastic waste pyrolysis oil over catalytic separation. Fuel 305(June):121440. https://doi.org/10.1016/j.fuel.2021.121440
Zhao Z, Qiu Z, Yang J, Lu S, Cao L, Zhang W, Xu Y (2017) Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy 167:183–188. https://doi.org/10.1016/j.hydromet.2016.11.013
Zhou J, Zhao J, Zhang J, Zhang T, Ye M, Liu Z (2020) Regeneration of catalysts deactivated by coke deposition: a review. Chin J Catal 41(7):1048–1061. https://doi.org/10.1016/S1872-2067(20)63552-5
Funding
Open access funding provided by Universitat Rovira i Virgili. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
PP contributed to conceptualization; CD and PP contributed to methodology; CD and PP contributed to software; CD and PP and DB contributed to validation; CM, DB and PP contributed to formal analysis; CD, PP and DB contributed to investigation; C. Medina, PP and DB contributed to resources; CD, PP and DB curated data; PP contributed to writing—original draft preparation; PP and JCB contributed to writing—review and editing; PP contributed to visualization; PP and JCB contributed to supervision; PP acquired funding. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palmay, P., Medina, C., Donoso, C. et al. Catalytic pyrolysis of recycled polypropylene using a regenerated FCC catalyst. Clean Techn Environ Policy 25, 1539–1549 (2023). https://doi.org/10.1007/s10098-022-02453-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-022-02453-4