Abstract
Since natural photosynthesis in our biosphere does not have the capacity to cope with the additional atmospheric CO2 due to combustion of fossil fuels, CO2 has to be actively removed. Efficient methods are currently being developed, but the captured gas has to be dumped in safe and permanent storage environments. Alternatively, it has to be purified before it can be recycled catalytically, using renewable energy, to high-value chemicals as feedstock for the synthesis of polymers, fine chemicals, or in large quantities liquid solar fuels. The combustion of solar fuels is carbon-neutral. If produced at locations where renewable energy is cheap, they become an important economic opportunity. The requirement to achieve a carbon-zero energy supply also for air traffic allows planning for an as yet unknown higher price compared to that of fossil fuels. Use of solar fuels in closed cycle applications may also relieve the energy situation in the large number of off-grid households in rural Africa. The availability of energy, in particular of electricity, is essential for advanced living conditions, prevents migration to urban areas, and therefore protects a rich variation of tribal cultural, religious and social traditions.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pre-industrial level of atmospheric CO2 amounts to about 280 ppm, which corresponds to 597 GtC (gigatons carbon) (Pachauri and Reisinger 2007). Currently, respiration of vegetation, soil and detritus add an annual amount of 196 Gt y−1, and outgassing of ocean water another 70.6 Gt y−1. This is compensated by roughly equal amounts of reabsorption by photosynthesis and uptake by the oceans (Pachauri and Reisinger 2007). Thus, nearly 50% of the atmospheric carbon is exchanged each year, which shows that nature is extremely efficient. Nevertheless, since the beginning of industrial evolution, the level of atmospheric CO2 has passed 400 ppm due to use of fossil fuels, which led to an additional annual emission that has meanwhile reached 6.4 Gt y−1. This comparatively small amount of only 2.5% of the amount of natural exchange could not be reabsorbed, not because nature is too slow but because the available vegetation limits its capacity. Therefore, even if emission of fossil CO2 is reduced to zero, the atmospheric concentration is not expected to decrease quickly unless we capture the excess actively (Stern 2007). This is not only necessary to cope with global warming but also to stop ocean acidification (Gruber et al. 2019).
There have been numerous suggestions for what to do with this captured CO2 “waste”. Three main alternatives are identified by the acronyms CCS (for carbon capture and storage), CCU (carbon capture and utilization) and BIO (biomass-grown and processed) (Gabrielli et al. 2020). CCS corresponds to the most common reaction of humans to waste, which is to dump it and bequeath the problem to our children. If captured at a point source and providing that the storage site has no leak, it prevents the emission of nearly 100% of the fossil carbon, and if CO2 is captured from the atmosphere, it is the only option that reduces its concentration permanently. CCU is an alternative to permanent storage that involves chemical recycling to value products or fuels. Sooner or later, this carbon will convert back to CO2, resulting in its multiple use, mimicking the natural carbon cycle and keeping the atmospheric concentration constant. The BIO route is essentially CCU that involves storing carbon by growing biomass (mainly forests) and reusing it by processing later. While this method captures CO2 efficiently from the atmosphere (Lewis et al. 2019) and stores it without much human intervention, it implies a long cycle time and a land capacity about 40 and 400 times larger than that required by the CCU and CCS routes, respectively (Gabrielli et al. 2020). It is therefore difficult to compare its economic aspects, and it will not be discussed here any further.
The amount of excess atmospheric carbon is huge and corresponds to 1.4 × 1016 mol of CO2, which equals 5.8 × 1011 m3 of gas at ambient temperature and 1 bar pressure, or 7.4 × 108 m3 of dry ice. It is therefore a formidable task to achieve a sufficient reduction of atmospheric CO2 as required by the Paris agreement on climate change (Paris agreement 2016) which aims at limiting global warming to 1.5–2 °C.
While the storage options are normally put in context with realistic economic and technical engineering procedures, we are of the opinion that the chemical processes of CO2 recycling remain too often within the fundamental science, without asking whether they will ever reach technical maturity. In the present work, we therefore focus on some of the principles that provide the necessary foundations for the technical feasibility of CO2 recycling to value chemicals and its energetic requirements and economic competitiveness to storage options. Moreover, the case of South Africa serves as a specific example for the motivation of CO2 conversion to liquid solar fuels.
The costs of carbon capture and storage
Prior to storage or utilization, CO2 has to be captured from the smoke stacks of fossil fuel power plants, or even better, from the atmosphere. Methods of air capture include adsorption/desorption cycles on porous molecular framework compounds functionalized with basic groups as pioneered by the start-up company Climeworks (Beuttler et al. 2019). Other approaches use amine-based functionalization of the adsorbent. This process yields a cost estimate (capture only) for fully developed industrial plants of US$ 100 per ton of CO2, corresponding to about US$ 0.25 per liter of burnt gasoline (CH2), depending on energy costs and other parameters. Alternatively, CO2 may be absorbed using aqueous potassium hydroxide (KOH) coupled to a caustic calcium recovery loop (Keith et al. 2018). A similar cost estimate as for the porous molecular framework compounds was projected for this method.
The most mature CCS storage technique is sequestration underground in deep saline aquifers or injection as pressurized supercritical CO2 with a density near 600 kg m−3 in depleted oil or gas reservoirs. A study of a natural 420,000-year-old CO2 reservoir in Arizona has shown a leakage rate of less than 0.01% per year (Miocic et al. 2019). The costs of CCS are quoted to amount to US$ 60–80 per ton of stored CO2 within the iron and steel industry and for refineries (Leeson et al. 2017), and leakage has been reported only once (Patel and Henriksen 2017). Alternatively, and perhaps more attractively, CO2 can be mineralized by reaction with rocks which are rich in calcium or magnesium. It is then stored in a stable, solid carbonate form, thereby mitigating health and environmental hazards. Various aspects of mineralization were evaluated and discussed in detail by Kelemen et al. (2019). The method can sequester large quantities (billions of tons) of CO2 per year without prior purification. Providing that suitable but unfortunately not so abundant porous geological formations such as basaltic lavas are available in close proximity, the method has been estimated to cost US$ 7–30 per ton of CO2 (storage costs only) which is relatively cheap compared with CCU methods and attractive for flue gases of fossil fuel power plants (Kelemen et al. 2019).
Fundamentals of technically promising CO2 recycling methods
While the above CCS method is cheap, it is a one-way deposition and not part of a circular economy since carbon is not reused and has to be replenished from fossil sources. Therefore, the interest in alternative (CCU) CO2 recycling has exploded, and diverse progress has been achieved over the past years, in particular regarding fundamental and mechanistic aspects (Gutiérrez Sánchez et al. 2019). A few points to highlight the prospects of large-scale application are as follows:
-
There is currently broad consensus that fossil fuels are not scarce on Earth (Gabrielli et al. 2020). Nevertheless, the primary economic principle must be the avoidance of CO2 emission since all options of recycling or sequestering CO2 lead to considerable additional cost (Sanedi 2008).
-
Nevertheless, even if no net fossil CO2 is emitted, we need to actively reduce the atmospheric CO2 for a transient period. Natural biological processes are very efficient in this, but they do not have the necessary capacity. Carbon must be permanently bound in order to reduce atmospheric CO2. A promising approach consists in planting trees. While this will increase the amount of bound carbon, the greening of our planet’s surface will increase absorption of solar radiation. It is therefore debatable whether forestation will also reduce climate warming (Popkin 2019; Chen et al. 2019).
-
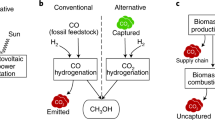
Using methanol as a representative example, its formation from coal is exothermic and exergonic but endothermic and endergonic from CO2 (Fig. 1). Using CO2 for the production of value chemicals is therefore naturally always more expensive than using coal because of the higher oxidation state of CO2. This has the consequence that green methanol is at present economically unattractive since it has a 1.3–2.6-fold higher cost compared to the current fossil-based analogue that costs US$ 0.63 per kg methanol. This is mainly due to the high price of hydrogen from water electrolysis, with up to 73% of the total cost (González-Garay et al. 2019). Purely short-term economic reasoning will therefore not enable us to solve the problem of global warming. However, the benefits of strong and early action to mitigate climate change far outweigh the economic costs of non-acting (Stern 2007).
-
Synthesizing methanol from CO2, trading it and converting it back to CO2 in a direct methanol fuel cell may serve as an example of a circular economy. Methanol formation by electrochemical conversion of CO2 in a polymer electrolyte electrolysis cell follows the equations
$$ \begin{aligned} &{\text{Anode}}: 3{\text{H}}_{2} {\text{O}}\,{\rightarrow}\, 3/2{\text{O}}_{2} + \, 6{\text{ H}}^{ + } + \, 6{\text{ e}}^{ - } \hfill \\ &{\text{Cathode}}: {\text{CO}}_{2} + \, 6{\text{H}}^{+} + \, 6{\text{e}}^{ - } \,{\rightarrow}\, {\text{CH}}_{3} {\text{OH }} + {\text{ H}}_{2} {\text{O}} \hfill \\ &{\text{Overall}}\;{\text{reaction}}:{\text{ CO}}_{2} + \, 3{\text{ H}}_{2} {\text{O}}\,{\rightarrow}\, {\text{CH}}_{3} {\text{OH }} + {\text{ H}}_{2} {\text{O }} + \, 3/2{\text{O}}_{2} \hfill \\ \end{aligned} $$(1)Since the oxygen cannot cross the membrane, it must be conserved separately in the anode and the cathode compartment, and one of the water molecules that is split at the anode is regenerated at the cathode, with an oxygen from CO2. Therefore, methanol forms in a 1:1 mixture with water and has to be separated if it is needed pure, as for addition to gasoline. This separation requires an additional effort, for example, through pervaporation through a suitable membrane (Senftle and Carter 2017). However, for use in a direct methanol fuel cell, the reaction is the reverse of reaction (1), and the 1:1 mixture can be used without separation.
-
Conversion processes have to be based on renewable energy, and since the prevalent renewable energy sources, photovoltaic and wind energy, are available in the form of electricity, it is vital to use electrochemical conversion techniques (Tatin, Bonin and Robert 2016). It has been pointed out that the main challenge of CCU cost reduction is not the CO2-to-fuel conversion step but the production of required carbon-free electricity at very low cost (Abanades et al. 2017). Fortunately, the price of solar and wind power is seen to be falling below US$ 0.03 per kWh in large parts of South Africa and neighboring countries (Sanedi 2008).
-
Current densities of technical interest on an industrial scale should approach values on the order of 1 A cm−2, which is common in fuel cells and electrolyzers. Such high values require the minimization of ohmic resistances and thus minimal separation distances between cathode and anode, which is realized best with proton– or OH–-conducting membranes. Furthermore, a cathode compartment that provides circulating gas phase CO2 avoids the low concentrations and the transport limitations of dissolved CO2 with concomitant depletion gradients in unstirred liquid electrolytes (Adegoke et al. 2020). Thus, a polymer electrolyte membrane water electrolysis cell with gas phase CO2 in the cathode compartment and with an anode catalyst that is active for CO2 reduction is expected to be the most promising configuration for large-scale applications (Vennekoetter et al. 2019).
-
For comparison, photovoltaics and artificial photosynthesis (the integrated one-step conversion of CO2 and water) have two major limitations. (1) The limited number of incident photons per unit area in unconcentrated solar light limits the maximum current density to on the order of 30 mA cm−2, depending on geographical latitude, daytime and seasonal variations. (2) Photovoltaic energy conversion is highly developed and occurs with close to maximum Shockley–Queisser efficiency of 30% for a solar cell with a bandgap of 1.1 eV (Shockley and Queisser 1961), while subsequent chemical conversions are still being improved. Catalytic surfaces in artificial photosynthesis have to be optimal photon converters and simultaneously optimal chemical catalysts, with a combined efficiency that will only in ideal cases approach the same values as for electrochemical conversion using electricity from standard photovoltaic cells.
-
Minimizing the cost of the product requires long-term stability of the electrodes, high product selectivity and a sufficient energy efficiency to warrant economic competitiveness. This requires electrolysis at the desired current densities at low overpotentials. It should also be emphasized that electrochemical processes are sensitive to impurities because of poisoning of the electrodes. This results in a key requirement for high purity gaseous reactants (flue gas purification), and crucially, water as a solvent and reactant.
Examples of CO2 electroreduction
While electrochemical reactions of large-scale industrial importance involve double electron transfer (fuel cell, water and chloralkali electrolysis), the reduction of CO2 to attractive products are multi-step reactions involving the transfer of often 4–8 or even more electrons. The electrocatalyst has to bind the reactant during all steps. The release of an intermediate renders it a product and determines the reaction selectivity.
The conventional belief that electrocatalytic activity requires classical metal or even transition metal electrodes has been superseded. Graphite and carbon glass have been used for simple reactions for a long time, but recent work has demonstrated that in particular nitrogen-doped graphene shows properties nearly identical to those of platinum for water splitting reactions (Zhen et al. 2014). Also oxides are used as cathode catalysts. Tin oxide is the most commonly used electrocatalyst for CO2 reduction, and a current density of 1 A cm−2 was obtained with a Faraday efficiency of 80% for formate (Löwe et al. 2019). Furthermore, indium oxide was reported to convert formic acid to a mixture of methanol, ethanol and iso-propanol with a combined Faraday efficiency up to 82% (Adegoke et al. 2020). A recent techno-economic analysis has concluded that under current conditions, carbon monoxide and formic acid are the only economically viable products of electrocatalytic CO2 conversion; however, higher-order alcohols such as ethanol and n-propanol could be highly promising under future conditions (Jouny et al. 2018).
Polyaniline (PANI)-based electrode materials were used for energy storage and conversion (Wang et al. 2016) and as conductive metal center support for CO2 electroreduction (Ponnurangam et al. 2017). Note that the conductive form of PANI is the partly oxidized form (emeraldine base or salt) (Song and Choi 2013), which needs to be considered when the material is applied in the reductive environment of the cathode reaction. It was demonstrated that biofunctionalized conductive polymers are able to reduce CO2 to CO (Coskun et al. 2017), and polyaniline films photocatalyze the CO2 reduction to alcohols (Hursán et al. 2016). Figure 2 proposes a mechanism where the amine moiety is the actual catalyst that binds CO2 in a conductive environment until a product (here methanol) is released after a multi-step reduction involving electron–proton transfer. The essential first step of CO2 binding is the formation of a carbamic acid. Importantly, in natural photosynthesis, the addition of CO2 to lysine 201 of rubisco to form the carbamate is essential for Mg2+ coordination and determines its catalytic activity (Berg et al. 2002). A similar mechanism with a polyamine as a capturing agent was proposed for one-step direct and quantitative thermal CO2 conversion to methanol from air capture, using hydrogen as a reductant and a homogeneous ruthenium catalyst (Kothandaraman et al. 2016).
Economic implications for South Africa
At present, more than 90% of South Africa’s electricity demand is produced from thermal power plants, the rest from nuclear and quite little hydroelectric, solar or wind energy. The recently updated Integrated Resource Plan 2019 (IRP 2019) represents a marked change to a future-oriented policy in which renewable energy sources receive significantly more attention. This is in line with the finding of a study that wind and solar energy are almost ideally complementary in South Africa in areas which are connected by the large national grid. The two renewable sources can take care of ca. 50% of the energy requirements without any additional storage (Knorr et al. 2016). However, the remaining vast sub-Saharan rural areas outside South Africa are to a large extent not connected to an electricity grid. They need a grid-independent energy strategy, for example involving solar fuels.
The coal-fired power plants currently account for half of the country’s CO2 emission. When considering the socioeconomic implications of the global climate change crisis on some developing countries in sub-Saharan Africa, the following needs to be addressed:
-
The increase in atmospheric CO2 concentration has taken place overwhelmingly by emission in the northern hemisphere, rather than in Africa or South America. Even though this part of the world is not responsible for climate change, it is most vulnerable to its impacts. The challenge is to reduce simultaneously inequality and greenhouse gas emission (Winkler 2018).
-
A country in financial duress cannot introduce carbon mitigating measures on its own accord without grave consequences to the stability of its economic and social structures. However, the proverbial opportunities in any crisis can be exploited for the good of the local and international community.
-
South Africa is unique in the world for its large-scale use of Fischer–Tropsch technology as a prime driver of its chemical and petrochemical industry. Its engineering and financial community could more readily be persuaded than others that this industry could in principle use synthesis gas (H2 and CO) from renewable energy and the electrolysis of CO2 and H2O in a solid oxide electrolysis cell (Kazempoor and Braun 2014) instead of emitting this CO2.. It would replace part of the synthesis gas from coal gasification in the existing setup. Against the backdrop of the global warming crisis, the continued use of natural gas and especially coal cannot be justified. The SASOL plant at Secunda is the world’s largest point source of CO2. Renewable liquid fuels, especially aviation fuels (Siegemund et al. 2017), will be sold at a price not directly related to present commercial values if the radical transition to a low-carbon future has to materialize. It will be determined by the best technology available for renewable fuels and what the industry can afford. This underlying uncertainty complicates detailed planning, also of research, when all the different strategies to reduce liquid fuels are considered. The advantage of liquid fuels is that in contrast to hydrogen gas, they are easy to transport over large distances with existing infrastructure, and they can be stored in large amounts to cope efficiently with peak energy requirements.
-
Rather than on the Fischer–Tropsch route, the future low-carbon chemical industry could, however, be more viable along the “methanol economy” route proposed by Nobel laureate Olah et al. (2006). Our reasoning is that a direct electrolytical production of methanol from CO2, H2O and renewable electricity, not yet as technically advanced as the electrolytic production of H2 and CO, may in future be more economically feasible because of its intrinsic simplicity. Methanol and its blends with traditional fuels can be used with minimal modification in petrol and diesel engines (Bechtold et al. 2007), but can also be the trading raw material for most of the chemical industry. If desired, gasoline or kerosene can be produced via the established methanol-to-gasoline process (Olsbye et al. 2012).
-
Methanol could be used to store large amounts of wind and solar energy for use in times of low generation, albeit with a lot less turnaround efficiency than the hydroelectric pumping schemes. Rather than in turbines or piston engines coupled to generators, methanol can also be used in a methanol fuel cell to produce electric energy directly from chemical energy, with a similar efficiency to that of the thermal power plants. Figure 3 illustrates this option, where part of sunny daytime renewable electricity can be stored in the form of methanol for back-conversion during the night or cloudy periods. Closed cycle operation retains the purified reactants and avoids poisoning of the electrocatalysts. The current bottlenecks are the energy efficiencies of methanol oxidation as well as of CO2 electroreduction. They are of intense interest to current research around the globe. A currently more mature and efficient alternative consists in closed cycle operation of water electrolysis and hydrogen fuel cell operation. Such closed cycle electrochemical processes are “confined” versions of large-scale circular economies according to Eq. (1).
Fig. 3 Operating scheme of complementary daytime photovoltaic and reduced power coal-fired supply of electrical power. A closed cycle methanol/CO2 interconversion permits energy storage in the form of liquid solar methanol during the day and its back-conversion during the night. Cyclic operation requires alternate storage of large amounts of CO2 and oxygen that could take place in large gas-tight inflatable tents or underground
-
Whether for the production of synthesis gas or methanol from CO2 and H2O, the electrocatalysts involved are at the center of research for long-life, robust, low overpotential energy-efficient and extremely selective reactions (Tatin et al. 2016), (Jouny et al. 2018). South Africa has a vested interest in creating markets for value-added noble metals mined in the world famous Merensky Reef (Barnes and Maier 2002). Catalyst development thus need not be limited to the more abundant elements, but should continue research into the Pt group metals and their efficient use in lower quantities and the accompanied challenges of recycling of spent catalysts.
-
From an African development perspective, the SA interest should include in its electrocatalytic research endeavors scalability as an aspirational criterion because, not only should we strive to drive the local economy with large-scale exports of renewable fuels, we should recognize the need for small scale, off-grid applications of liquid fuels and energy storage media. This strategy would support the late UN Secretary General Kofi Annan’s dream of skipping a generation of technology for Africa’s development (Anan 2015). Providing renewable electricity is essential to the health of off-grid communities since refrigeration permits the preservation of food and medical drugs, and it provides the energy for water purification. Moreover, it allows for wireless communication with the world and for access to information via TV and internet. This would relieve the pressure for migration from rural areas to large urban centers, and it would permit keeping the rich cultural diversity in large parts of Africa.
-
Whatever route electrocatalytic technology takes for the storage and transport of renewable energy, whether for small- or large-scale applications: a mature technology will finally produce fuel largely determined by the price of renewable electric energy. This is where South Africa will have a major economic advantage because of its large, arid and low-populated geographical regions suitable for wind and solar energy production (Wright et al. 2019). The rest of the world therefore has an interest in the early development of such local infrastructure toward the future production of affordable transport fuels, especially for aviation, where the highest power-to-mass ratio of carbon-based fuels is of overriding interest (Siegemund et al. 2017).
Change history
21 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10098-021-02136-6
References
Abanades JC, Rubin ES, Mazzotti M, Herzog HJ (2017) On the climate change mitigation potential of CO2 conversion to fuels. Energy Environ Sci 10:2491–2499
Adegoke KA, Radhakrishnan SG, Gray CL, Sowa B, Morais C, Rayess P, Rohwer ER, Comminges C, Kokoh KB, Roduner E (2020) Highly efficient formic acid and carbon dioxide electro-reduction to alcohols on indium oxide electrodes. Sust Energy Fuels. https://doi.org/10.1039/D0SE00623H
Anan K (2015) Foreword in: Africa progress report 2015: Seizing Africa’s energy and climate opportunities. Africa Progress Panel. ISBN 978-2-9700821-6-3
Barnes SJ, Maier WD (2002) Platinum-group elements and microstructures of normal Merensky reef from Impala Platinum Mines, Bushveld complex. J Petrol 43:103–128. https://doi.org/10.1093/petrology/43.1.103
Bechtold RL, Goodman MB, Timbario TA (2007) Use of methanol as a transportation fuel. http://www.methanol.org/wp-content/uploads/2016/06/Methanol-Use-in-Transportation.pdf
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. 5th edition. W. H. Freeman, New York, Section 20.2. ISBN-10: 0-7167-3051-0
Beuttler C, Charles L, Wurzbacher J (2019) The role of direct air capture in mitigation of anthropogenic greenhouse gas emissions. Front Clim. https://doi.org/10.3389/fclim.2019.00010
Chen C, Park T, Wang X, Piao S, Xu B, Chaturvedi RK, Fuchs R, Brovkin V, Ciais P, Fensholt R, Tømmervik H, Bala G, Zhu Z, Nemani RR, Myneni RB (2019) China and India lead in greening of the world through land-use management. Nat Sustain 2:122–129
Coskun H, Aljabour A, De Luna P, Farka D, Greunz T, Stifter D, Kus M, Zheng X, Liu M, Hassel AW, Schöfberger W, Sargent EH, Sariciftci NS, Stadler P (2017) Biofunctionalized conductive polymers enable efficient CO2 electroreduction. Sci Adv 3:e1700686
Gabrielli P, Gazzani M, Mazzotti M (2020) The role of carbon capture and utilization, carbon capture and storage, and biomass to enable a net-zero-CO2 emissions chemical Industry. Ind Eng Chem Res 59:7033–7045
González-Garay A, Frei MS, Al-Qahtani A, Mondelli C, Guillén-Gonsálbez G, Pérez-Ramirez J (2019) Planet-to-planet analysis of CO2-based methanol processes. Energy Environ Sci 12:3425–3436
Gruber N, Clement D, Carter BR, Feely RA, van Heuven S, Hoppema M, Ishii M, Key RM, Kozyr A, Lauvset SK, Monaco CL, Mathis JT, Murata A, Olsen A, Perez FF, Sabine CL, Tanhua T, Wanninkhof R (2019) The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363:1193–1199
Gutiérrez Sánchez O, Birdja YY, Bulut M, Vaes J, Breugelmans T, Pant D (2019) Recent advances in industrial CO2 electroreduction. Curr Op Green and Sust Chem 16:47–55
Hursán D, Kormányos A, Rajeshwar K, Janáky C (2016) Polyaniline films photoelectrochemically reduce CO2 to alcohols. Chem Commun 52:8858–8861
IRP (2019) Integrated resource plan. Department of Energy, Republic of South Africa http://www.energy.gov.za/IRP/2019/IRP-2019.pdf(downloaded 15.06.2020)
Jouny K, Luc W, Jiao F (2018) General techno-economic analysis of CO2 electrolysis systems. Ind Eng Chem Res 57:2165–2177
Kazempoor P, Braun RJ (2014) Performance analysis of solid oxide electrolysis cells for syngas production. ECS Trans 58:43–53
Keith DW, Holmes G, Angelo D, Heidel K (2018) A process for capturing CO2 from the atmosphere. Joule 2:1–22
Kelemen P, Benson SM, Pilorgé H, Psarra P, Wilcox J (2019) An overview of the status and challenges of CO2 storage in minerals and geological formations. Front Clim. https://doi.org/10.3389/fclim.2019.00009
Knorr K, Zimmermann B, Bofinger S, Gerlach A-K, Bischof-Niemz T, Mushwana C (2016) Wind and solar PV resource aggregation study for South Africa. https://www.csir.co.za/sites/default/files/Documents/Wind%20and%20Solar%20PV%20Resource%20Aggregation%20Study%20for%20South%20Africa_Final%20report.pdf(downloaded 15.06.2020)
Kothandaraman J, Goeppert A, Czaun M, Olah GA, Surya Prakash GK (2016) Conversion of CO2 from Air into methanol using a polyamine and a homogeneous ruthenium catalyst. J Am Chem Soc 138:778–781
Leeson D, Mac Dowell N, Shah N, Petit C, Fennell PS (2017) A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int J Greenhouse Gas Control 61:71–84
Lewis SL, Wheeler CE, Mitchard ETA, Koch A (2019) Regenerate natural forests to store carbon. Nature 568:25–28
Löwe A, Rieg C, Hierlemann T, Salas N, Kpljar D, Wagner N, Klemm E (2019) Influence of temperature on the performance of gas diffusion electrodes in the CO2 reduction reaction. ChemElectroChem 6:4497–4506
Miocic JM, Gilfillan SMV, Frank NN, Schroeder-Ritzrau A, Burnside NM, Haszeldine RS (2019) 420,000 year assessment of fault leakage rates shows geological carbon storage is secure. Sci Rep 9:769
Olah GA, Goeppert A, Surya Prakash GA (2006) Beyond oil and gas: the methanol economy. WILEY-VCH, Weinheim. ISBN 3-527-31275-7
Olsbye U, Svelle S, Bjørgen M, Beato P, Janssens TVW, Joensen F, Bordiga S, Lillerud KP (2012) Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity. Angew Chem Int Ed 51:5810–5831
Pachauri RK, Reisinger A (Eds). Intergovernmental panel on climate change, Assessment report 4 (IPCC AR4), Geneva, 2007. https://www.ipcc.ch/publications_and_data/ar4/wg1/en/figure-7-3.html. Accessed 18 August 2016
Paris agreement (2016). https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement(downloaded 08.06.2020)
Patel P, Henriksen PP (2017) Can carbon capture and storage deliver on its promise? MRS Bullet 42:188–189
Ponnurangam S, Chernyshova IV, Somasundaran P (2017) Nitrogen-containing polymers as a platform for CO2 electroreduction. Adv Coll Interface Sci 244:184–198
Popkin G (2019) The forest question. Nature 565:280–282
Sanedi (2008) Analysis of CO2 utilisation technologies and their suitability for implementation in South Africa. South African National Energy Development Institute. https://www.sacccs.org.za/Reports/(downloaded 10.06.2020)
Senftle TP, Carter EA (2017) The holy grail: chemistry enabling an economically viable CO2 capture, utilization, and storage strategy. Acc Chem Res 50:472–475
Shockley W, Queisser HJ (1961) Detailed balance limit of efficiency of p-n junction solar cells. J Appl Phys 32:510–519
Siegemund S, Trommler M, Kolb O, Zinnecker V (2017) The potential of electricity-based fuels for low-emission transport in the EU. German Energy Agency (dena), Berlin
Song E, Choi J-W (2013) Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomat 3:498–523. https://doi.org/10.3390/nano3030498
Stern N (2007) The economics of climate change. The Stern review. Cambridge University Press, Cambridge
Tatin A, Bonin J, Robert M (2016) A case for electrofuels. ACS Energy Lett 1:1062–1064
Vennekoetter J-B, Sengpiel R, Wessling M (2019) Beyond the catalyst: how electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem Eng J 364:89–101
Wang H, Lin J, Shen ZX (2016) Polyaniline (PANI) based electrode materials for energy storage and conversion. J Sci Adv Mater Dev 1:225e225
Winkler H (2018) Reducing inequality and carbon emissions: innovation and developmental pathways. South Afr J Sci 114:1–7
Wright JG, Bischof-Niemz T, Calitz JR, Mushwana C, van Heerden R (2019) Long-term electricity sector expansion planning: a unique opportunity for a least cost energy transition in South Africa. Renew Energy Focus 30:21–45
Zhen Y, Jiao Y, Zhu Y, Li LH, Han Y, Chen Y, Du A, Jaroniec M, Qiao SZ (2014) Hydrogen evolution by a metal-free electrocatalyst. Nat Commun 5:3783
Funding
Support by the University of Pretoria and by the NRF Grant 87401 (Swiss South African Joint Research Project (SSAJRP)) is gratefully acknowledged. We thank for stimulating discussions and collaboration with Dr. Artur Braun from the Empa in Switzerland. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.R.R. initiated the project and wrote chapter 5. E.R. coordinated the work and wrote chapters 1–4.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Invited contribution to special issue on “Circular economy: national and global policy”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roduner, E., Rohwer, E.R. Technical principles of atmospheric carbon dioxide reduction and conversion: economic considerations for some developing countries. Clean Techn Environ Policy 23, 475–482 (2021). https://doi.org/10.1007/s10098-020-01889-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-020-01889-w