Abstract
A simple low-temperature co-precipitation method was employed to synthesize pure and Ba-doped self-assembled ZnO nanospheres. The presence of self-assembled nanosphere-like morphology, high crystallinity, uniform size distribution, and more defects were confirmed by X-ray diffraction, high-resolution scanning electron microscopy, high-resolution transmission electron microscopy, diffuse reflectance spectroscopy, and photoluminescence spectroscopy. Photocatalytic degradation of 2,4,6-trichlorophenol, a potent endocrine-disrupting chemical in aqueous medium was investigated. The effects of Ba doping on the structure, morphology, absorption, emission, and photocatalytic activity of ZnO nanospheres were investigated systematically. Furthermore, the effects of different photocatalytic degradation reaction parameters were investigated. The resulting photocatalytic degradation activity of Ba-doped ZnO nanospheres shows superior efficiency compared to pure ZnO and commercial TiO2 (Degussa P-25).
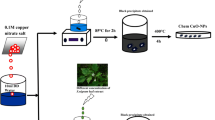
Graphical Abstract
A simple co-precipitation method was developed to synthesize pure and Ba-doped ZnO self-assembled nanospheres. The prepared photocatalyst shows a novel morphology, high crystallinity, uniform size distribution, and more defects. Effect of Ba doping on the structural, optical and photocatalytic properties of ZnO nanospheres were investigated systematically.
















Similar content being viewed by others
References
Anandan S, Vinu A, Venkatachalam N, Arabindoo B, Murugesan V (2006) Photocatalytic activity of ZnO impregnated Hβ and mechanical mix of ZnO/Hβ in the degradation of monocrotophos in aqueous solution. J Mol Catal A 256:312–320
Arruda LB, Orlandi MO, Filho PNL (2013) Morphological modifications and surface amorphization in ZnO sonochemically treated nanoparticles. Ultrason Sonochem 20:799–804
Ayyub P, Palkar VR, Chattopadhyay S, Multani M (1995) Effect of crystal size reduction on lattice symmetry and cooperative properties. Phys Rev B 51:6135–6138
Becker J, Raghupathi KR, Pierre JS, Zhao D, Koodali RT (2011) Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J Phys Chem C 115:13844–13850
Bohle DS, Spina CJ (2009) Cationic and anionic surface binding sites on nanocrystalline zinc oxide: Surface influence on photoluminescence and photocatalysis. J Am Chem Soc 131:4397–4404
Borges ME, Herna´ndez T, Esparza P (2014) Photocatalysis as a potential tertiary treatment of urban wastewater: new photocatalytic materials. Clean Technol Environ Policy 16:431–436
Bougrine A, Hichou AE, Addou M, Ebothe J, Kachouane A, Troyon M (2003) Structural, optical and cathodoluminescence characteristics of undoped and tin-doped ZnO thin films prepared by spray pyrolysis. Mater Chem Phys 80:438–445
Diaz GD, Garcia MC, Lopez MCB, Castanon MJL, Ordieres AJM, Blanco PT (2010) Heterogeneous catalytic 2,4,6-trichlorophenol degradation at hemin–acrylic copolymer. Appl Catal B 96:51–56
Dodd AC, McKinley AJ, Saunders M, Tsuzuki T (2006) Effect of particle size on the photocatalytic activity of nanoparticulate zinc oxide. J Nanopart Res 8:43–51
Dong JY, Hsu YJ, Wong DSH, Lu SY (2010) Growth of ZnO nanostructures with controllable morphology using a facile green antisolvent method. J Phys Chem C 114:8867–8872
Gao R, Wang J (2007) Effects of pH and temperature on isotherm parameters of chlorophenols biosorption to anaerobic granular sludge. J Hazard Mater 145:398–403
Gultekin I, Ince NH (2007) Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J Environ Manag 85:816–832
Huang MH, Wu Y, Feick H, Tran N, Weber E, Yang P (2001) Catalytic growth of zinc oxide nanowires by vapor transport. Adv Mater 13:113–116
Janisch R, Gopal P, Spaldin NA (2005) Transition metal-doped TiO2 and ZnO-present status of the field. J Phys 17:R657–R689
Janotti A, Walle CGVD (2009) Fundamentals of zinc oxide as a semiconductor. Rep Prog Phys 72:126501
Kanade KG, Kale BB, Aiyer RC, Das BK (2006) Effect of solvents on the synthesis of nano-size zinc oxide and its properties. Mater Res Bull 41:590–600
Khalilzadeh A, Fatemi S (2014) Modification of nano-TiO2 by doping with nitrogen and fluorine and study acetaldehyde removal under visible light irradiation. Clean Technol Environ Policy 16:629–636
Mahdavi S (2015) Nano-TiO2 modified with natural and chemical compounds as efficient adsorbents for the removal of Cd+2, Cu+2, and Ni+2 from water. Clean Technol Environ Policy. doi:10.1007/s10098-015-0993-y
Meenatchisundaram S, Devaraj M, Rai CL, Nadarajan KM (2014) An integrated approach for enhanced textile dye degradation by pre-treatment combined biodegradation. Clean Technol Environ Policy 16:501–511
Pardeshi SK, Patil AB (2009) Solar photocatalytic degradation of resorcinol a model endocrine distrupter in water using zinc oxide. J Hazard Mater 163:403–409
Porte C, Janer G, Lorusso LC, Zarragoitia MO, Cajaraville MP, Fossi MC, Canesi L (2006) Endocrine disruptors in marine organisms: approaches and perspectives. Comp Biochem Physiol Part C 143:303–315
Sabbaghan M, Firooz AA, Ahmadi VJ (2012) The effect of template on morphology, optical and photocatalytic properties of ZnO nanostructures. J Mol Liq 175:135–140
Sakthivel S, Shankar MV, Palanichamy M, Arabindoo B, Bahnemann DW, Murugesan V (2004) Enhancement of photocatalytic activity by metal deposition: characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res 38:3001–3008
Selvam NCS, Vijaya JJ, Kennedy LJ (2012) Effects of morphology and Zr doping on structural, optical, and photocatalytic properties of ZnO nanostructures. Ind Eng Chem Res 51:16333–16345
Selvam NCS, Jesudoss SK, Rajan PI, Kennedy LJ, Vijaya JJ (2015) Comparative investigation on the photocatalytic degradation of 2,4,6-trichlorophenol using pure and M-doped (M=Ba, Ce, Mg) ZnO spherical nanoparticles. J Nanosci Nanotechnol 15:5910–5917
Serpone N, Lawless D, Disdier J, Herrmann JM (1994) Spectroscopic, photoconductivity, and photocatalytic studies of TiO2 colloids: naked and with the lattice doped with Cr3+, Fe3+, and V5+ cations. Langmuir 10:643–652
Shirgholami MA, Nazari A, Mirjalili M (2015) Statistical optimization of self-cleaning technology and color reduction in wool fabric by nano zinc oxide and eco-friendly cross-linker. Clean Technol Environ Policy 17:905–919
Sin J, Lam S, Mohamed AR, Lee K (2012) Degrading endocrine disrupting chemicals from wastewater by TiO2 photocatalysis: a review. Int J Photoenergy 2012:23. doi:10.1155/2012/185159
Sun J, Qiao L, Sun S, Wang G (2008) Photocatalytic degradation of orange G on nitrogen: doped TiO2 catalysts under visible light and sunlight irradiation. J Hazard Mater 155:312–319
Tang Q, Zhou W, Shen J, Zhang W, Kong L, Qian Y (2004) A template-free aqueous route to ZnO nanorod arrays with high optical property. Chem Commun 10:712–713
Titus MP, Molina VG, Banos MA, Gimenez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B Environ 47:219–256
Vanhesuden K, Warren WL, Voigt JA, Seager CH, Tallant DR (1995) Impact of Pb doping on the optical and electronic properties of ZnO powders. Appl Phys Lett 67:1280
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys 16:R829–R858
Wang C, Zhang WX, Qian XF, Zhang XM, Xie Y, Qian YT (1999) A room temperature chemical route to nanocrystalline PbS semiconductor. Mater Lett 40:255–258
Wang R, Xin JH, Yang Y, Liu H, Xu L, Hu J (2004) The characteristics and photocatalytic activities of silver doped ZnO nanocrystallite. Appl Surf Sci 227:312–317
Wang H, Xie C, Zhang W, Cai S, Yang Z, Gui Y (2007) Comparison of dye degradation efficiency using ZnO powders with various size scales. J Hazard Mater 141:645–652
Yoffre AD (1993) Low-dimensional systems: quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv Phys 42:173–266
Zheng Y, Chen C, Zhan Y, Lin X, Zheng Q, Wei K (2007) Luminescence and photocatalytic activity of ZnO nanocrystals: correlation between structure and property. Inorg Chem 46:6675–6682
Acknowledgments
The authors duly acknowledge the financial support rendered by Loyola college, Tamil Nadu, India under Loyola College–Times of India (LC-TOI) Major Research Project scheme (Project code: 2LCTOI14CHM003, dated November, 25, 2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jesudoss, S.K., Vijaya, J.J., Selvam, N.C.S. et al. Effects of Ba doping on structural, morphological, optical, and photocatalytic properties of self-assembled ZnO nanospheres. Clean Techn Environ Policy 18, 729–741 (2016). https://doi.org/10.1007/s10098-015-1047-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-1047-1