Abstract
Purpose
The objective of this multicenter study was to compare the diagnostic performance of lateral flow assay (LFA) and enzyme-linked immunosorbent assay (ELISA) to detect the Dynamiker Aspergillus Galactomannan levels in serum and bronchoalveolar lavage fluid (BALF) samples for I.
Methods
We registered 310 clinically suspected Aspergillus infection patients from December 2021 to February 2023 and classified them into subgroups as the “IA group” and “non-IA group” based on the latest EORTC/MSG guidelines. The immunoassays were analyzed by LFA and ELISA respectively.
Results
Galactomannan was examined using LFA, and serum and BALF samples demonstrated sensitivities of 82.57% and 89.47%, specificities of 90.76% and 92.00%, PPVs of 89.11% and 96.23%, and NPVs of 85.04% and 79.31%, respectively. Galactomannan was observed using two assays in serum and BALF samples and showed PPAs of 95.11% and 93.33%, NPAs of 89.19% and 96.30%, and TPAs of 92.47% and 94.25%, respectively. The ROC curve demonstrated that LFA had optimum diagnostic value when the index value (I value) = 0.5, the sensitivity was 84.94%, and the specificity was 90.97%.
Conclusion
Compared to the ELISA method, the LFA has shown excellent performance for the diagnosis of IA in serum and BALF sample and can be used as an assay for the early diagnosis of patients with IA. The dynamic change in galactomannan levels may be useful for assessing treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to cutting-edge research by Fudan University in Shanghai, China, and the Global Action Fund for Fungal Infections (GAFFI), as many as 300,000 people worldwide are affected by invasive aspergillosis (IA), and currently, an estimated 1,179,000 people are affected by IA in China alone. This study did not include COVID-19 and influenza cases, two additional risk factors for IA [1, 2].
People at risk of IA and chronic aspergillosis included 774,000 patients with lung cancer; 29,000,000 patients hospitalized for chronic obstructive pulmonary diseases (COPDs) such as emphysema; 844,000 patients with pulmonary tuberculosis (PTB); 41,200 patients with acute myelogenous leukemia; 106,000 patients with advanced AIDS; and 21,400 transplant recipients[3, 4]. The incidence of IA is very high in patients with severe influenza and COVID-19, but it is difficult to estimate the incidence because the number of affected patients varies from year to year. IA is a leading cause of death in acute leukemia and bone marrow transplant patients. Timely initiation of antifungal therapy can determine life or death in these patients.
Aspergillus galactomannan is a polysaccharide component widely found in the cell wall of Aspergillus. After Aspergillus invades lung tissue, early galactomannan is released in body fluids and blood, such as BALF, cerebrospinal fluid, and pleural fluid. Galactomannan levels can be tested in blood or body fluids before clinical symptoms and signs appear in IA patients, so the galactomannan test is a prominent tool in the early diagnosis of IA[5, 6]. The most frequently used method for antigen detection is the enzyme-linked immunosorbent assay (ELISA), the results of which can be reported within 2 h. In the EORTC/MSG guidelines, galactomannan tests are advocated for early and rapid diagnosis [7].
Several new approaches have also been developed, one of which is the LFA. An LFA is an independent immunochromatographic assay similar to a home pregnancy test for the qualitative and semiquantitative testing of galactomannan in serum and BALF samples; the test produces results in approximately 20 min and has good concordance with ELISA [8, 9]. Early and fast diagnosis of IA followed by targeted antifungal therapy has the potential to significantly improve survival. Continuous galactomannan testing is an option for IA testing in these patients, and ELISA and LFA commercial kits have been validated in some clinical studies [10].
The objective of this multicenter study was to determine the effectiveness of a novel QuicGM™ Aspergillus galactomannan Ag LFA produced by Dynamiker Biotechnology (Tianjin) Co., Ltd., used as a screening test for IA. QuicGM™ LFA is a fluorescent immunochromatographic test kit using monoclonal antibodies against galactomannan and europium nanoparticles. It is a semiquantitative assay combined with a portable detection device that can be widely accepted by the clinical and primary medical communities for the early and fast diagnosis of IA. A comparative study of LFA and ELISA was performed to validate the performance of this new assay.
Materials and methods
Study design and population
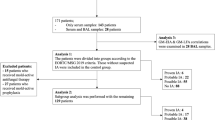
We organized a prospective multicenter cohort study at three medical centers, including Ruijin Hospital affiliated with the Shanghai Jiao Tong University School of Medicine, Shanghai East Hospital affiliated with the Tongji University School of Medicine, and Qilu Hospital of Shandong University. We registered 310 patients with clinically suspected Aspergillus infection from December 2021 to February 2023. The EORTC/MSG has developed definitions for the diagnosis of possible, probable, and proven IA. These definitions depend on host criteria (immunosuppressive conditions), clinical criteria (clinical and radiological signs of IPA), and mycological criteria (results of direct or indirect microbiological testing for Aspergillus species). Patients who did not conform to the definition were defined as non-IA. Ruijin Hospital approved this study (code RJ2022246) before commencement.
LFA and ELISA analysis
Two immunoassays were used for the semiquantitative testing of Aspergillus galactomannan antigen, including the Dynamiker QuicGM™ Aspergillus galactomannan lateral flow assay (LFA) and the Dynamiker Aspergillus Galactomannan enzyme-linked immunosorbent assay (ELISA). According to the manufacturer’s instructions, a serum/BALF sample I value ≥ 0.5 was considered a positive result for LFA and ELISA.
For the LFA, 300 μL serum/BALF samples were pretreated by the addition of 100 μL sample treatment solution in 1.5-mL screw-cap polypropylene tubes, vortexed for 10 s, and centrifuged at 10,000 × g for 15 s to shake off the sample of the centrifuge tube. Depending on the laboratory apparatus, the sample was heated for 5 min in a water bath or heat block and then centrifuged at 10,000 × g for 10 min. A total of 90–100 μL of sample supernatant was transferred into the sample well. The I value results were recorded with the fluorescence immunoassay analyzer provided by the manufacturer after 20 min.
For ELISA, the sample pretreatment method was consistent with that for the LFA. Then, 100 μL of supernatant was added to microtiter wells, incubated at 37 °C for 60 min, and washed. Next, 100 μL of the conjugate was added to microtiter wells, incubated at 37 °C for 30 min, and washed. Then, 100 μL of substrate solution was added to microtiter wells and incubated at 37 °C for 25 min. The stopping solution was added to microtiter wells and incubated at 37 °C for 5 min to terminate the reaction. The OD at 450 nm (reference 620/630 nm) was read within 5 min after the addition of the stopping solution (Fig. 1).
Statistical analyses
The clinical performance of the two assays was validated in the studied population by calculating the sensitivity, specificity, positive predictive value, and negative predictive value. ROC curve analysis was used to determine the overall performance in the studied population and the best positive threshold to improve the specificity and sensitivity of the two assays. Spearman’s rank correlation coefficient was used to determine the quantitative agreement between the two assays. The Mann–Whitney test in paired analysis and the Kruskal–Wallis test were used to compare multiple median values for different groups. Statistics were performed by using GraphPad Prism 8.0.2, and P < 0.05 was considered significant.
Results
Patient characteristics
In total, 419 samples from 310 patients (166 patients in the proven/probable IA group, 144 patients in the non-IA group) were tested, including 332 serum samples and 87 BALF samples.
The patient characteristics and underlying diseases in this study are summarized in Table 1. Compared with the non-IA group, the number of males in the IA group was higher, but the difference was not statistically significant (P > 0.01). The non-IA group was older than the IA group (P < 0.01). The common underlying diseases in IA patients were diabetes mellitus, sepsis, and acute myeloid leukemia. The most common symptoms in IA patients were cough, sputum, and fever. Chest pain, dyspnea, and hemoptysis were not common in IA patients.
Fig. 2 shows representative chest computed tomography (CT) images of some cases. In these IA patients, specific chest CT findings, such as the air crescent sign and halo sign, were rare. The chest CT findings showed that the incidence of single or multiple nodules was consistent with other reports [11].
Representative examples of chest CT findings in IA patients. A Aspergillus nodules in the right upper and lower lobes and left lower lobe. B An Aspergillus nodule with pleural effusion in the left lobe. C Consolidation in the right middle lobe and left upper lobe. D Bronchiectasis in the right lower and middle lobes. E An Aspergillus nodule with cavitary lesions in the right middle lobe.
Aspergillus spp. were isolated from eight patients. One of the patients was infected with both A. terreus and A. nidulans. Aspergillus spp. were tested in seven patients by mNGS. One of the patients was infected with A. fumigatus, A. terreus, and A. flavus. The differences in the median GM I value in the serum and BALF samples between the IA group and the non-IA group were significant (P < 0.0001 and 0.0022).
There was no significant difference in the C-reactive protein (CRP) concentration in peripheral blood between the IA group and the non-IA group (P > 0.05). The IA group showed significantly higher levels of interleukin-6 (IL-6) and procalcitonin (PCT) in peripheral blood than the non-IA group (P < 0.05, Fig. 3). Previous studies confirmed that IL-6 and PCT concentrations were positively correlated with galactomannan levels. This result suggests that the diagnostic potential of more cytokines for IA should be tested, and their binding with other IA biomarkers should be evaluated [12,13,14].
Percent agreement of LFA and ELISA
In serum sample, BALF samples, and total samples, the total percent agreement (TPA) between LFA and ELISA was 92.47%, 94.25%, and 92.84%, respectively, representing good agreement.
The positive percent agreement (PPA) and negative percent agreement (NPA) for serum samples were 95.11% and 89.19%, respectively, with most of the discordance arising due to samples that were negative by LFA but positive by ELISA. The PPA and NPA for BALF samples were 93.33% and 96.30% (Table 2), respectively.
Concordance between LFA and ELISA
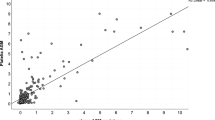
The semiquantitative correlation between the GM I values calculated by LFA and ELISA was excellent (Spearman’s coefficient, r = 0.9022). For serum samples, the semiquantitative correlation between the GM I values for LFA and ELISA was better than that for BALF samples (Spearman’s coefficient, r = 0.9064 vs. r = 0.8805, Fig. 4).
Analysis of detection levels
The median levels detected by LFA in serum samples from the IA group were significantly higher than those detected by ELISA (P = 0.006), but there was no significant difference in BALF samples between LFA and ELISA (P = 0.081). In the non-IA patient samples, there was no significant difference in serum and BALF samples between LFA and ELISA (P = 0.3232 and 0.7567). The detection levels of the two assays are summarized in Fig. 5.
Lymphopenia, neutropenia, and CD4 T cells are essential factors in immunocompromised patients. In the study by Cordonnier et al., there was a significant difference in GM levels between neutropenic patients and nonneutropenic patients [15]. According to some studies, neutropenia is a considerable risk factor for fungal infections.
In the study by Susianti et al., there were no significant differences in the total leucocyte count and neutrophil count in the fungal-infected group and nonfungal-infected group [16]. Our results are consistent with the literature in that there were no significant differences in GM I values among IA patients with and without neutropenia (P > 0.05). There were no significant differences in the GM I values in IA patients with and without leukopenia (P > 0.05, Fig. 6).
Performance of LFA and ELISA
At the manufacturer’s recommended GM I value of 0.5, the sensitivity and specificity of LFA were 82.57% and 90.76% in serum samples and 89.47% and 92.00% in BALF samples, respectively. The sensitivity and specificity of ELISA in serum samples and BALF samples were greater, as shown in Table 3. ROC analysis confirmed the best LFA I values to be 0.4 and 0.5 in serum and BALF samples, respectively (Fig. 7). ROC analysis confirmed the best ELISA optimal positivity threshold to be 0.51 and 0.50 in serum and BALF samples, respectively. The performances of GM (LFA/ELISA) for diagnosing IA at the best I value in different samples are shown in Table 4.
Kinetic profile analysis of LFA and ELISA
We selected three patients and analyzed all of their GM test results. At an I value of 0.5, LFA and ELISA could monitor the GM levels in the serum of IA patients and non-IA patients (Fig. 8). Continuous dynamic monitoring of high-risk patients has value in early diagnosis and confirms that monitoring the dynamic change in serum GM content is also conducive to the judgment of the treatment effect and the development of the disease.
Discussion
In this study, the performance of a galactomannan antigen kit (LFA) with an associated automated fluorescence immunoassay analyzer was evaluated on BALF and serum samples for the diagnosis of IA. The patients were mainly from the respiratory, ICU, and hematology departments. The LFA is considered an important innovation in the mycological sciences. In particular, the usefulness of the test and its early results, high sensitivity and specificity, and ability to work in body fluids other than BALF or serum are important advantages. Some studies have reported on the practicality of the IMMY Aspergillus LFA in serum or BALF [10, 17, 18].
The diagnostic performance of LFA and ELISA correlated well in our study. In our analysis, with an optimized cutoff of 0.5 as recommended by the manufacturer, the sensitivity of LFA for the diagnosis of IA in serum samples was 82.57%, the specificity was 90.76%, the PPA was 89.11%, and the NPA was 85.04%. These findings are consistent with the previously published results by Serin et al., in which LFA had a sensitivity of 90.9% and a specificity of 90.8% for an I value of 0.5 in predicting IA [19]. In the study by Almeida-Paes et al., the sensitivity of LFA in serum was less than that of ELISA (74% versus 89%) [20]. In a study by Linder et al., BALF GM LFA had poor sensitivity but very high specificity for the diagnosis of IA [21]. We also observed that both ELISA and LFA GM in BALF samples had high specificity for the diagnosis of IA (92.00% versus 92.00%).
In the study by White et al., a qualitative agreement between the IMMY LFA and Bio-Rad ELISA was excellent in case and control populations [22]. In our study, the qualitative agreement between the LFA and ELISA was excellent in both serum and BALF samples. In serum samples, the PPA between the GM LFA and ELISA was 95.11% better than that in BALF samples (93.33%). In BALF samples, the NPA between LFA and ELISA was 96.30%, which was better than that in serum samples (89.19). The semiquantitative correlation between the GM I value for case-based samples was excellent (Spearman’s coefficient, r = 0.9022).
Other sources of infection could not be excluded from our subjects, so it was difficult to find patients with only fungal infections. Therefore, our study showed that many fungal-infected patients had leukocytosis and neutrophilia. There were no significant differences in GM levels in patients with and without neutropenia. The number of neutropenia samples was rare, so there are some limitations in this study. Hence, our results indicate that BALF is superior to serum in the detection of IA by the ELISA/LFA method. Monitoring the GM levels in IA patients undergoing antifungal treatment showed that GM showed a gradual decrease in patients in remission. The LFA and ELISA results were consistent. These results are consistent with a previous report documenting that GM levels could be used to monitor treatment response in patients with IA[23].
In conclusion, the Dynamiker LFA provides a comparable alternative to ELISA when testing serum and BALF samples. When using the usual 0.5 threshold, the LFA performance was slightly inferior to that of the ELISA companion assay. We found that the BALF Aspergillus GM LFA had high sensitivity and specificity for the diagnosis of IA. The test system was simple to use, and the results were highly reproducible.
Data availability
The data sets generated during the current study are available from the corresponding authors upon reasonable request.
References
Global Action Fund for Fungal Infections (2022) Country fungal disease burdens. https://gaffi.org/media/country-fungal-disease-burdens/.n.d. Accessed 7 Oct 2022
Zhou L-H, Jiang Y-K, Li R-Y et al (2020) Risk-based estimate of human fungal disease burden, China. Emerg Infect Dis 26:2137–2147. https://doi.org/10.3201/eid2609.200016
Chinese State Statistical Bureau (2018) China statistical yearbook. http://www.stats.gov.cn/tjsj/ndsj/2018/indexch.htm.n.d. Accessed 7 Oct 2022
Wang C, Xu J, Yang L et al (2018) Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 391:1706–1717. https://doi.org/10.1016/S0140-6736(18)30841-9
Bassetti M, Giacobbe DR, Grecchi C et al (2020) Performance of existing definitions and tests for the diagnosis of invasive aspergillosis in critically ill, adult patients: a systematic review with qualitative evidence synthesis. J Infect 81:131–146. https://doi.org/10.1016/j.jinf.2020.03.065
Lamoth F (2016) Galactomannan and 1,3-β-d-glucan testing for the diagnosis of invasive aspergillosis. J Fungi 2:22. https://doi.org/10.3390/jof2030022
Donnelly JP, Chen SC, Kauffman CA et al (2020) Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367–1376. https://doi.org/10.1093/cid/ciz1008
Jenks JD, Hoenigl M (2020) Point-of-care diagnostics for invasive aspergillosis: nearing the finish line. Expert Rev Mol Diagn 20:1009–1017. https://doi.org/10.1080/14737159.2020.1820864
Jenks JD, Mehta SR, Taplitz R et al (2019) Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus Galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycoses 62:230–236. https://doi.org/10.1111/myc.12881
White PL, Price JS, Posso R et al (2020) Evaluation of the performance of the IMMY sona Aspergillus Galactomannan lateral flow assay when testing serum to aid in diagnosis of invasive aspergillosis. J Clin Microbiol 58:e00053–e00020. https://doi.org/10.1128/JCM.00053-20
Zhang L, Che C (2019) Clinical manifestations and outcome analysis of invasive pulmonary aspergillosis infection: a retrospective study in 43 nonneutropenic patients. J Int Med Res 47:5680–5688. https://doi.org/10.1177/0300060519874901
Liu F, Zhang X, Du W et al (2021) Diagnosis values of IL-6 and IL-8 levels in serum and bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. J Investig Med 69:1344–1349. https://doi.org/10.1136/jim-2021-001857
Zhong Y, Ji T, Qin D, Cheng D (2023) Clinical characteristics and risk factors of in-hospital mortality in patients coinfected with Pneumocystis jirovecii and Aspergillus. J Med Mycol 33:101330. https://doi.org/10.1016/j.mycmed.2022.101330
Heldt S, Eigl S, Prattes J et al (2017) Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses 60:818–825. https://doi.org/10.1111/myc.12679
Cordonnier C, Botterel F, Ben Amor R et al (2009) Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect 15:81–86. https://doi.org/10.1111/j.1469-0691.2008.02122.x
Susianti H, Parmadi L, Firani NK et al (2021) Diagnostic value of serum human Galactomannan aspergillus antigen and 1,3-beta-D-glucan in immunocompromised patient suspected fungal infection. J Clin Lab Anal 35:e23806. https://doi.org/10.1002/jcla.23806
Jenks JD, Mehta SR, Taplitz R et al (2019) Bronchoalveolar lavage Aspergillus Galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test for diagnosis of invasive pulmonary Aspergillosis in patients with hematological malignancies. J Infect 78:249–259. https://doi.org/10.1016/j.jinf.2018.10.014
Mercier T, Dunbar A, de Kort E et al (2020) Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: a comparative multicenter study. Med Mycol 58:444–452. https://doi.org/10.1093/mmy/myz079
Serin I, Dogu MH (2021) Serum Aspergillus galactomannan lateral flow assay for the diagnosis of invasive aspergillosis: a single-centre study. Mycoses 64:678–683. https://doi.org/10.1111/myc.13265
Almeida-Paes R, Almeida MD, de Macedo PM et al (2022) Performance of two commercial assays for the detection of serum aspergillus galactomannan in non-neutropenic patients. J Fungi 8:741. https://doi.org/10.3390/jof8070741
Linder KA, Kauffman CA, Miceli MH (2020) Performance of Aspergillus Galactomannan lateral flow assay on bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis. J Fungi 6:297. https://doi.org/10.3390/jof6040297
White PL, Price JS, Posso R et al (2020) An evaluation of the performance of the IMMY Aspergillus Galactomannan enzyme-linked immunosorbent assay when testing serum to aid in the diagnosis of invasive aspergillosis. J Clin Microbiol 58:e01006–e01020. https://doi.org/10.1128/JCM.01006-20
Patterson TF, Thompson GR, Denning DW et al (2016) Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. https://doi.org/10.1093/cid/ciw326
Funding
This study was supported by the Shanghai Key Public Health Discipline (grant number GWV-10.1-XK04), Shanghai Excellent Technology Leader (grant number 20XD1434500), and the Shandong Key Research and Development Program (2019GSF108130).
Author information
Authors and Affiliations
Contributions
JG and WL: conception and design. WT: data analysis. DQ and JG: revising the work and manuscript writing. DW, LH, LN, and LL: collection and assembly of data. WW and WL: final approval of the manuscript and agreement to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (codeRJ2022246). This study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
A waiver of informed consent was granted by the committee owing to the use of remnant and de-identifed samples for the study. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Xiao, C., Tian, W. et al. Performance of the Aspergillus galactomannan lateral flow assay with a digital reader for the diagnosis of invasive aspergillosis: a multicenter study. Eur J Clin Microbiol Infect Dis 43, 249–257 (2024). https://doi.org/10.1007/s10096-023-04724-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04724-4