Abstract
Purpose
To characterize the resistance mechanisms affecting the cefepime-taniborbactam combination in a collection of carbapenemase-producing Enterobacterales (CPE) and carbapenem-resistant Pseudomonas spp. (predominantly P. aeruginosa; CRPA) clinical isolates.
Methods
CPE (n = 247) and CRPA (n = 170) isolates were prospectively collected from patients admitted to 8 Spanish hospitals. Susceptibility to cefepime-taniborbactam and comparators was determined by broth microdilution. Cefepime-taniborbactam was the most active agent, inhibiting 97.6% of CPE and 67.1% of CRPA (MICs ≤ 8/4 mg/L). All isolates with cefepime-taniborbactam MIC > 8/4 mg/L (5 CPE and 52 CRPA) and a subset with MIC ≤ 8/4 mg/L (23 CPE and 24 CRPA) were characterized by whole genome sequencing.
Results
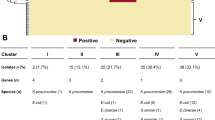
A reduced cefepime-taniborbactam activity was found in two KPC-ST307-Klebsiella pneumoniae isolates with altered porins [KPC-62-K. pneumoniae (OmpA, OmpR/EnvZ), KPC-150-K. pneumoniae (OmpK35, OmpK36)] and one each ST133-VIM-1-Enterobacter hormaechei with altered OmpD, OmpR, and OmpC; IMP-8-ST24-Enterobacter asburiae; and NDM-5-Escherichia coli with an YRIN-inserted PBP3 and a mutated PBP2. Among the P. aeruginosa (68/76), elevated cefepime-taniborbactam MICs were mostly associated with GES-5-ST235, OXA-2+VIM-2-ST235, and OXA-2+VIM-20-ST175 isolates also carrying mutations in PBP3, efflux pump (mexR, mexZ) and AmpC (mpl) regulators, and non-carbapenemase-ST175 isolates with AmpD-T139M and PBP3-R504C mutations. Overall, accumulation of these mutations was frequently detected among non-carbapenemase producers.
Conclusions
The reduced cefepime-taniborbactam activity among the minority of isolates with elevated cefepime-taniborbactam MICs is not only due to IMP carbapenemases but also to the accumulation of multiple resistance mechanisms, including PBP and porin mutations in CPE and chromosomal mutations leading to efflux pumps up-regulation, AmpC overexpression, and PBP modifications in P. aeruginosa.


Similar content being viewed by others
Data Availability
The sequences generated in this project were submitted to the European Nucleotide Archive under the study accession number PRJNA985214.
References
Nordmann P, Naas T, Poirel L (2011) Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3310682&tool=pmcentrez&rendertype=abstract10.3201/eid1710.110655
Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S et al (2019) Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 32:1–52. https://doi.org/10.1128/CMR.00031-19
Botelho J, Grosso F, Peixe L (2019) Antibiotic resistance in Pseudomonas aeruginosa – mechanisms, epidemiology and evolution. Drug Resist Updat 44:26–47. https://doi.org/10.1016/j.drup.2019.07.002
Yahav D, Giske CG, Gramatniece A, Abodakpi H, Tam VH, Leibovici L (2021) New β-lactam–β-lactamase inhibitor combinations. Clin Microbiol Rev 1(34):1–61. https://doi.org/10.1128/CMR.00115-20
Vázquez-Ucha JC, Arca-Suárez J, Bou G, Beceiro A (2020) New carbapenemase inhibitors: clearing the way for the β-lactams. Int J Mol Sci 1(21):9308. https://doi.org/10.3390/ijms21239308
Liu B, Trout REL, Chu GH, Mcgarry D, Jackson RW, Hamrick JC et al (2020) Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem 26(63):2789–2801. https://doi.org/10.1021/acs.jmedchem.9b01518
Hamrick JC, Docquier J-D, Uehara T, Myers CL, Six DA, Chatwin CL et al (2020) VNRX-5133 (Taniborbactam), a broad-spectrum inhibitor of serine-and metallo-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01963–e01919
Krajnc A, Brem J, Hinchliffe P, Calvopiña K, Panduwawala TD, Lang PA et al (2019) Bicyclic boronate VNRX-5133 inhibits metallo- and serine-β-lactamases. J Med Chem 26(62):8544–8556. https://doi.org/10.1021/acs.jmedchem.9b00911
Hernández-García M, García-Castillo M, Ruiz-Garbajosa P, Bou G, Siller-Ruiz M, Pitart C, Gracia-Ahufinger I et al (2022) In vitro activity of cefepime-taniborbactam against carbapenemase-producing Enterobacterales and Pseudomonas aeruginosa isolates recovered in Spain. Antimicrob Agents Chemother 66(3):e0216121. https://doi.org/10.1128/aac.02161-21
Hernández-García M, García-Castillo M, García-Fernández S, Melo-Cristino J, Pinto MF, Goncalves E et al (2020) Distinct epidemiology and resistance mechanisms affecting ceftolozane / tazobactam in Pseudomonas aeruginosa isolates recovered from ICU patients in Spain and Portugal depicted by WGS. J Antimicrob Chemother 1–10. https://doi.org/10.1093/jac/dkaa430
Hernández-García M, García-fernández S, García-castillo M, Melo-Cristino J, Pinto MF, Goncalves E et al (2020) Confronting ceftolozane-tazobactam susceptibility in multidrug-resistant Enterobacterales isolates and whole-genome sequencing results (STEP study). Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.106259
Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S et al (2016) Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol 17:1–14. https://doi.org/10.1186/s13059-016-0997-x
Beghain J, Bridier-Nahmias A, Le NH, Denamur E, Clermont O (2018) ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb Genom 4:1–8. https://doi.org/10.1099/mgen.0.000192
Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE (2021) A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun 12:4188. https://doi.org/10.1038/s41467-021-24448-3
Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM (2017) PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother 72:2764–2768. https://doi.org/10.1093/jac/dkx217
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL et al (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Karlowsky JA, Hackel MA, Wise MG, Six DA, Uehara T, Daigle DM et al (2023) In vitro activity of cefepime-taniborbactam and comparators against clinical isolates of gram-negative bacilli from 2018 to 2020: results from the Global Evaluation of Antimicrobial Resistance via Surveillance (GEARS) Program. Antimicrob Agents Chemother 1:67. https://doi.org/10.1128/aac.01281-22
Shields RK, Chen L, Cheng S, Chavda KD, Press EG (2016) Emergence of ceftazidime-avibactam resistance fue to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:1–11. https://doi.org/10.1128/AAC.02097-16
Bianco G, Boattini M, Comini S, Iannaccone M, Bondi A, Cavallo R et al (2022) In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis 1(41):63–70. https://doi.org/10.1007/s10096-021-04341-z
Daigle D, Hamrick J, Chatwin C, Kurepina N, Kreiswirth BN, Shields RK, Oliver A, Clancy CJ, Nguyen MH, Pevear D, Xerri L (2018) Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug-resistant K. pneumoniae and P. aeruginosa. Open Forum Infect Dis Ther 5(Suppl):1
Castillo-Polo JA, Hernández-García M, Morosini MI, Pérez-Viso B, Soriano C, De Pablo R et al (2023) Outbreak by KPC-62-producing ST307 Klebsiella pneumoniae isolates resistant to ceftazidime/avibactam and cefiderocol in a university hospital in Madrid, Spain. J Antimicrob Chemother 25. https://doi.org/10.1093/jac/dkad086
Satapoomin N, Dulyayangkul P, Avison MB (2022) Klebsiella pneumoniae mutants resistant to ceftazidime- avibactam plus aztreonam, imipenem-relebactam, meropenem-vaborbactam, and cefepime-taniborbactam. Antimicrob Agents Chemother 1:66. https://doi.org/10.1128/aac.02179-21
Alm RA, Johnstone MR, Lahiri SD (2014) Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: Role of a novel insertion in PBP3. J Antimicrob Chemother 20(70):1420–1428. https://doi.org/10.1093/jac/dku568
Sato T, Ito A, Ishioka Y, Matsumoto S, Rokushima M, Kazmierczak KM et al (2020) Escherichia coli strains possessing a four amino acid YRIN insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC Antimicrob Resist 1:2. https://doi.org/10.1093/jacamr/dlaa081
Wang X, Zhao C, Wang Q, Wang Z, Liang X, Zhang F et al (2020) In vitro activity of the novel β-lactamase inhibitor taniborbactam (VNRX-5133), in combination with cefepime or meropenem, against MDR Gram-negative bacterial isolates from China. J Antimicrob Chemother 1(75):1850–1858. https://doi.org/10.1093/jac/dkaa053
Ranjitkar S, Reck F, Ke X, Zhu Q, McEnroe G, Lopez SL et al (2019) Identification of mutations in the mrdA gene encoding PBP2 that reduce carbapenem and diazabicyclooctane susceptibility of Escherichia coli clinical isolates with mutations in ftsI (PBP3) and which carry blaNDM-1. mSphere 28:4. https://doi.org/10.1128/msphere.00074-19
Vázquez-Ucha JC, Lasarte-Monterrubio C, Guijarro-Sánchez P, Oviaño M, Álvarez-Fraga L, Alonso-García I et al (2022) Assessment of activity and resistance mechanisms to cefepime in combination with the novel β-lactamase inhibitors zidebactam, taniborbactam, and enmetazobactam against a multicenter collection of carbapenemase-producing Enterobacterales. Antimicrob Agents Chemother 1:66. https://doi.org/10.1128/AAC.01676-21
Le Terrier C, Nordmann P, Sadek M, Poirel L (2023) In vitro activity of cefepime/zidebactam and cefepime/taniborbactam against aztreonam/avibactam-resistant NDM-like-producing Escherichia coli clinical isolates. J Antimicrob Chemother 1(78):1191–1194. https://doi.org/10.1093/jac/dkad061
Golden AR, Baxter MR, Karlowsky JA, Mataseje L, Mulvey MR, Walkty A et al (2022) Activity of cefepime/taniborbactam and comparators against whole genome sequenced ertapenem-non-susceptible Enterobacterales clinical isolates: CANWARD 2007-19. JAC Antimicrob Resist 1:4. https://doi.org/10.1093/jacamr/dlab197
Le Terrier C, Nordmann P, Freret C, Seigneur M, Poirel L (2023) Impact of acquired broad spectrum b-lactamases on susceptibility to novel combinations made of β-lactams (aztreonam, cefepime, meropenem, and imipenem) and novel β-lactamase inhibitors in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 1:67. https://doi.org/10.1128/aac.00339-23
Lasarte-Monterrubio C, Fraile-Ribot PA, Vázquez-Ucha JC, Cabot G, Guijarro-Sánchez P, Alonso-García I et al (2022) Activity of cefiderocol, imipenem/relebactam, cefepime/taniborbactam and cefepime/zidebactam against ceftolozane/tazobactam- and ceftazidime/avibactam-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 1(77):2809–2815. https://doi.org/10.1093/jac/dkac241
Pérez-Vázquez M, Sola-Campoy PJ, Zurita ÁM, Ávila A, Gómez-Bertomeu F, Solís S et al (2020) Carbapenemase-producing Pseudomonas aeruginosa in Spain: interregional dissemination of the high-risk clones ST175 and ST244 carrying blaVIM-2, blaVIM-1, blaIMP-8, blaVIM-20 and blaKPC-2. Int J Antimicrob Agents 1(56). https://doi.org/10.1016/j.ijantimicag.2020.106026
Hernández-García M, García-Castillo M, Melo-Cristino J, Pinto MF, Gonçalves E, Alves V et al (2022) In vitro activity of imipenem/relebactam against Pseudomonas aeruginosa isolates recovered from ICU patients in Spain and Portugal (SUPERIOR and STEP studies). J Antimicrob Chemother 1(77):3163–3172. https://doi.org/10.1093/jac/dkac298
López-Causapé C, Cabot G, del Barrio-Tofiño E, Oliver A (2018) The versatile mutational resistome of Pseudomonas aeruginosa. Front Microbiol 6:9. https://doi.org/10.3389/fmicb.2018.00685
Acknowledgements
The study group includes the following members: Germán Bou and M. Carmen Fernández, Hospital Universitario A Coruña, A Coruña, Spain; Jorge Calvo, Jesús Rodríguez-Lozano, and María Siller-Ruiz, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Jordi Vila and Cristina Pitart, Hospital Clínic i Provincial, Barcelona, Spain; Luis Martínez-Martínez and Irene Gracia-Ahufinger, Hospital Universitario Reina Sofía, Córdoba, Spain; Antonio Oliver and Xavier Mulet, Hospital Universitario Son Espases, Palma de Mallorca, Spain; Álvaro Pascual and Elena Marín-Martínez, Hospital Universitario Virgen Macarena, Sevilla, Spain; Concepción Gimeno and Nuria Tormo, Consorcio Hospital General Universitario de Valencia, Valencia, Spain; Marta Hernández-García, María García del Castillo, Patricia Ruiz-Garbajosa, Marta Nieto-Torres, and Rafael Cantón, Hospital Ramón y Cajal, Madrid, Spain.
Funding
This study was also supported by Plan Nacional de I + D + i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases [RD16/0016/0001, RD16/0016/0004, RD16/0016/0006, RD16/0016/0007, RD16/0016/0008, RD16/0016/0010, and REIPI RD16/0016/0011], CIBER de Enfermedades Infecciosas (CIBERINFEC) (CB21/13/00084), and co-financed by the European Development Regional Fund “A way to achieve Europe” (ERDF), Operative program Intelligent Growth 2014–2020. MH-G is supported by a postdoctoral contract by CIBERINFEC (CB21/13/00084).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The ethics committees of Ramón y Cajal University hospital approved the study (Ref. 038-20).
Conflict of interest
RC has participated in educational programs organized by MSD, Pfizer, and Shionogi, and has received research support from MSD and Venatorx Pharmaceuticals, Inc. Other authors do not declare conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández-García, M., García-Castillo, M., Nieto-Torres, M. et al. Deciphering mechanisms affecting cefepime-taniborbactam in vitro activity in carbapenemase-producing Enterobacterales and carbapenem-resistant Pseudomonas spp. isolates recovered during a surveillance study in Spain. Eur J Clin Microbiol Infect Dis 43, 279–296 (2024). https://doi.org/10.1007/s10096-023-04697-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04697-4