Abstract
Purpose
Due to its ability to disseminate worldwide and its multiple resistance trait, Acinetobacter baumannii is becoming a threat for public health worldwide. Cefiderocol (FDC) is a promising broad-spectrum cephalosporin recently approved for treating Gram-negative infection. The aim of this study was to develop a rapid test, namely the rapid FDC Acinetobacter baumannii NP test, for the detection of FDC susceptibility/resistance in A. baumannii since the current FDC susceptibility tests are rather time-consuming (at least 24 h).
Materials and methods
The rapid test is based on the reduction of resazurin to resorufin product by bacterial viable cells, thus detecting bacterial growth in the presence of FDC (38.4 mg/L). A color change from blue (resazurin) to violet or pink (resorufin) represents visual detection of bacterial growth. 95 randomly selected A. baumannii isolates were used to evaluate the performance of the rapid FDC Acinetobacter baumannii NP test.
Results
The test showed 95.5% (95% CI 78.2–99.2%) and 100.0% (95% CI 95.0–100.0%) of sensitivity and specificity, respectively. All the results were obtained within 4 h30–4 h45 incubation time at 35 °C ± 2 °C, saving virtually one day when compared with currently-used antimicrobial susceptibility tests. The test showed only a single very major error, an isolate with a MIC of 8 mg/L.
Conclusions
The rapid FDC Acinetobacter baumannii NP test can be a valuable method which is easier and faster to interpret when compared with the gold standard broth microdilution method. The test showed remarkable performances; hence, it may be suitable for implementation in clinical microbiology routine laboratories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii is a gram-negative opportunistic pathogen responsible for causing a series of healthcare-associated infections that tend to occur in patients hospitalized in intensive care units and includes pneumonia, bloodstream, urinary, and wound infections [1]. Those infections are difficult to treat and are often combined with longer hospitalization and high mortality rates, generating a great concern for public health [2].
Carbapenems are frequently one important choice for treating these infections; however, outbreaks and reports of carbapenem-resistant A. baumannii (CRAB) are already disseminated globally [3]. Consequently, in 2017, the World Health Organization (WHO) included CRABs as “critical” in their list of priority pathogens for research and development of new antibiotics [4]. Carbapenem resistance in A. baumannii has been related to distinct mechanisms such as outer membrane permeability defects (porins), overexpression of genes encoding efflux pumps, and modification of penicillin-binding proteins (PBPs), but mostly through production of acquired carbapenemases [(KPC Ambler class A), IMP, NDM, VIM (class B), and OXA-23, OXA-24/40, OXA-58, and OXA-72 (class D)] and also through the overexpression of the blaOXA-51-like gene being intrinsic to this species [2].
Among the last treatment options, colistin and polymyxin B usage is frequently associated with acute kidney injury, and, like tigecycline, both have limited penetration in infection sites such as lung and urine [5]. Furthermore, the novel sulbactam-durlobactam combination does not inhibit the strains producing class B metallo-β-lactamases (MBL) [6]. Hence, there is an urgent need to search and develop new drugs for treating A. baumannii infections. FDC is a siderophore cephalosporin which is able to bind to extracellular free iron via its siderophore side chain, allowing active transport into the periplasmic space of gram-negative bacteria through siderophore uptake systems, and, in addition, is also able to invade bacterial cells by passive diffusion through outer membrane porin channels [7].
FDC has demonstrated in vitro activity against the majority of β-lactamases, including MBLs, and has been approved by the FDA and EMA, respectively, in 2019 and 2020, for the treatment of infections due to aerobic gram-negative microorganisms in adults with complicated urinary tract infections (cUTI), including pyelonephritis caused by susceptible strains [7, 8]. Although its recent approval for use in humans and its limited usage, isolates of CRAB resistant to FDC have been reported and associated with PER-like β-lactamases [9], NDM-like β-lactamases [9], point mutations in PBP3 and OXA-23 [10], and piuA (TonB-dependent siderophore receptor) or pirA (siderophore gene) [11].
The broth microdilution (BMD) method, the standard reference for determining susceptibility to A. baumannii, is rather time-consuming, and interpretation of the results is quite debatable and challenging for a microbiology routine laboratory [12]. Other antimicrobial susceptibility techniques such as disk diffusion methods (30 μg FDC discs, Liofilchem, Roseto degli Abruzzi, Italy) and UMIC® Cefiderocol (Bruker Daltonics, Bremen, Germany) can be used as an alternative, but they still require at least 18 h to obtain the results. Moreover, currently, the FDA (susceptible ≤ 1; intermediate: 2; resistant > 4 mg/L) [13], the CLSI (susceptible ≤ 4; intermediate: 8; resistant > 16 mg/L) [14], and the EUCAST (susceptible ≤ 2; resistant > 2 mg/L) [15] have different breakpoints for interpreting FDC susceptibility results for A. baumannii, turning its interpretation even more challenging.
Thus, the development of an inexpensive, rapid, precise, and trustful test for the detection of FDC resistance in A. baumannii may be beneficial to optimize the treatment of patients infected by A. baumannii. The rapid FDC Acinetobacter baumannii NP test was therefore designed for that purpose.
Materials and methods
Bacterial isolates and antimicrobial susceptibility testing
95 A. baumannii isolates were randomly selected from the collection of the Swiss National Reference Center of Emerging Antibiotic Resistance (NARA). Isolates from this collection were previously sent to the reference center for investigation of their carbapenemase content. The main β-lactamase gene as a source of the multidrug resistance pattern of all isolates had been previously characterized, and it is shown on Table 1.
The BMD reference method was performed to determine the minimum inhibitory concentration (MIC) of all isolates following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [14]. Results were interpreted according to the pharmacokinetic/pharmacodynamic (PK/PD) breakpoints from EUCAST (susceptible ≤ 2 mg/L; resistant > 2 mg/L) [15, 16]. BMD was considered the gold standard when comparing to the results obtained with the rapid FDC Acinetobacter baumannii NP test. The reference strain A. baumannii CIP 70.10 (ATCC 15151) was used as quality control for both techniques, and the reference Pseudomonas aeruginosa (ATCC 27853) strain was also used for BMD quality control.
Optimizing rapid FDC Acinetobacter baumannii NP test
The rapid test is based on the reduction of resazurin (a viability colorant) to resorufin product by bacterial viable cells, thus detecting bacterial growth in the presence of a fixed concentration of FDC. Bacterial growth is visually detected by a color change from blue (resazurin) to violet or pink (resorufin).
In order to reach optimal conditions of the test, several different parameters were tested using the FDC-susceptible A. baumannii CIP 70.10 and one resistant isolate with a MIC ≥ 128 mg/L. These parameters comprised variable FDC concentrations (8, 16, 24.8, 32, 38.4, 46.2, 64, and 128 mg/L), variable bacterial inoculum concentrations (0.5 of the McFarland’s scale, 1/5, 1/10, 1/15, 1/20, 1/25 of the 1.0 McFarland’s scale), different bacterial inoculum volumes (20 and 50 µL), different reagent solution volume (150 and 180 µL), and variable incubation times (4 h, 4h15, 4h30, 4h45, and 5 h).
The optimal conditions were FDC final concentration at 38.4 mg/L, inoculum concentration of 1/20 of the 1.0 McFarland’s scale, bacterial inoculum volume of 20 µL, reagent solution volume of 180 µL, and 4h30-4h45 of incubation time.
Preparation of the rapid FDC Acinetobacter baumannii NP solution
The solution was prepared with iron-depleted Mueller–Hinton broth (ID-MHB; chelex 100 resin, Bio-Rad, Marnes-la-Coquette, France; MHB, AxonLab, Baden, Switzerland) [14] supplemented or not with FDC (Shionogi, Osaka, Japan) at a concentration of 42.67 mg/L targeting to achieve a final fixed concentration of 38.4 mg/L in a 200 µL final volume in each microplate’s well. The solution can be stored at − 80 °C for 2 weeks.
Bacterial inoculum
Overnight cultures were grown on UriSelect 4 (Bio-Rad, Marnes-la-Coquette, France). After that, a 1.0 McFarland bacterial inoculum was prepared by adding bacterial colonies in 5 mL sterile NaCl (0.85%) and then diluting 1/20 (50 µL of inoculum in 950 µL of NaCl) before inoculation into the microplates in a range from 15 min to 1 h after preparation [15].
Tray inoculation
A 96-well polystyrene microplate (round base, with lid, sterile; Sarstedt, Nuembrecht, Germany) was used to perform the rapid FDC Acinetobacter baumannii NP test. The bacterial suspension was inoculated in two independent wells, with and without FDC. The steps to perform the test were as follows: (1) 180 µL of FDC-free solution was added to wells A1-A4; (2) 180 µL of FDC at a concentration of 42.67 mg/L was added to wells B1-B4; (3) 20 µL (1/20 of the 1.0 McFarland scale) of A. baumannii CIP 70.10 (negative control) was added to wells A1 and B1; (4) 20 µL of a FDC-resistant isolate (positive control) was added to wells A2 and B2; (5) 20 µL of a tested isolate was added to wells A3 and B3; and (6) 20 µL of NaCl 0.85% was added to wells A4 and B4 (Fig. 1). After preparing the microplate for the test and before inoculating the bacterial suspensions, the rapid FDC Acinetobacter baumannii NP solution was pre-warmed at 37 °C for 15–30 min before use to prevent growth delay and therefore a delayed color change.
The rapid FDC Acinetobacter baumannii NP test. Column A presents the solution free of FDC. Column B presents the solution with FDC (38.4 mg/L). Reference strain A. baumannii CIP 70.10 (ATCC 15151) was inoculated in A1 and B1; FDC-resistant isolate (positive control) was inoculated in A2 and B2; tested isolate (resistant to FDC) that grew in both absence and presence of FDC was inoculated in A3 and B3; and NaCl 0.85% was inoculated in A4 and B4 as control of contamination and possible spontaneous color change. Bacterial growth is evidenced by a color change of the medium from blue to pink or violet
Tray incubation and reading
The tray was not sealed and the test was incubated for 3 h at 35 ± 2 °C without shaking. After that time, 20 µL of resazurin reagent PrestoBlue (Thermo Fisher Scientific, Cleveland, OH, USA) was added to each well [(concentration of 10% (vol/vol)] and the test was incubated again. Reading was performed visually by checking the tray for no spontaneous color change after 4 h and then every 15 min until reaching a total of 4h45 h of incubation. The test was considered positive when the isolate grew (color change from blue to pink or violet) in the presence of FDC, and negative when no growth was observed (no color change) in the presence of FDC. Figure 1 provides a comprehensive illustration of the visual interpretation of the rapid FDC Acinetobacter baumannii NP test.
To assure optimal quality control of the test and consequently validate the rapid FDC Acinetobacter baumannii NP test, the following conditions had to be reached; (1) blue-to-pink/violet color change observed, confirming the bacterial growth and viability for all isolates in wells without FDC (A1-A3); (2) no color change observed (remaining blue) in wells after adding NaCl 0.85%, confirming the absence of contamination (A4 and B4); (3) blue to pink/violet color changes observed in the wells where the positive control and the tested isolate were added (A2 and B2; A3 and B3); and (4) no color change observed with the reference strain A. baumannii CIP 70.10 in the well with FDC (negative control) (B1). See Fig. 1 for some examples.
Data analysis
Discrepancies between the rapid FDC Acinetobacter baumannii NP test and the BMD standard reference method were determined and classified, if present, as very major errors (VME) and major errors (ME) as previously described [17, 18]. Sensitivity, specificity, accuracy, and precision parameters were also calculated to evaluate the performance of the test proposed. Results were blindly read and interpreted independently by two laboratory members.
Results
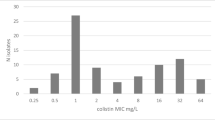
The tested collection comprised 95 randomly-selected A. baumannii isolates including OXA-23 (n = 78 [82.1%]), OXA-24/40 (n = 5 [5.26%]), OXA-26 (n = 1 [1.05%]), OXA-58 (n = 4 [4.2%]), OXA-72 (n = 1 [1.05%]), GES-12 (n = 1 [1.05%]), IMP-4 (n = 1 [1.05%]), NDM-1 (n = 1 [1.05%]), OXA-23 co-production with NDM-like (NDM-1 and NDM-5) (n = 1 [1.05%] for each one), and AmpC ADC overexpressed (n = 1 [1.05%]). A total of 73 (76.8%) of these isolates were susceptible (MIC range: ≤ 0.125 to 2 mg/L) and 22 (23.2%) were resistant (MIC range: 4 to ≥ 128 mg/L) to FDC according to the BMD results and interpreted following the EUCAST guidelines [15]. Those results are summarized in Table 1.
All the FDC-susceptible isolates were negative, and 21 FDC-resistant isolates gave positive results for the rapid FDC Acinetobacter baumannii NP test. Only a single resistant isolate with an MIC of FDC at 8 mg/L, being an OXA-23 producer, gave a negative result for the test (Table 1). This was the only VME (false negative) result observed with the test, although no ME (false positive) was identified. The test showed 95.5% (95% CI 78.2–99.2%) of sensitivity, 100.0% (95% CI 95.0–100.0%) of specificity, 98.9% of accuracy, and 100% of precision when compared with the BMD standard method. After incubating for 4 h (1 h after resazurin was added), the color change of the wells was read at every 15 min, and it was concluded that the optimal reading of the final results shall be at 4h30-4h45 after incubation at 35 °C ± 2 °C under an ambient atmosphere.
Discussion
A. baumannii, especially when resistant to carbapenems, is a challenging threat to hospitalized patients once they are able to, not only cause multidrug resistant infections, but also to survive in facility surfaces and shared medical equipment, being a perfect candidate for nosocomial outbreaks. Treating those infections is difficult and frequently ends in failures. The promising FDC molecule is a notable treatment alternative for CRAB infections since it is stable against the hydrolysis activity of ESBLs, class A and D carbapenemases, class C cephalosporinases, and most MBLs [5].
In the present study, an original and accurate test has been developed for the detection of FDC susceptibility/resistance in A. baumannii that showed excellent sensitivity and specificity. The rapid FDC Acinetobacter baumannii NP test was able to detect FDC susceptibility/resistance within 4h30-4h45, saving approximately 14 h (a day in practice) from the currently available antimicrobial susceptibility tests, including the reference standard BMD. In addition, it is rather easier for interpreting the results, once is only a matter of visually detecting the viable cells growing in cefiderocol by a color change from blue (resazurin) to violet or pink (resorufin), instead of the controversial existence of trailing endpoints that challenges and difficult BMD interpretation for this antibiotic.
The rapid FDC Acinetobacter baumannii NP test showed very few discrepancies when compared with the BMD (no ME and only one VME). Moreover, the proposed test revealed a significant correlation with the standard technique in terms of sensitivity, specificity, accuracy, and precision parameters. The only VME identified in this study had an MIC of 8 mg/L and may be related to the slow metabolism of that particular isolate. Of note, a limitation of the present study is that a limited number of FDC-resistant isolates were available to be tested, once this profile remains relatively scarce. Therefore, further testing in other laboratories will be required for assessing the exact performances of the test.
Also notable is that the EUCAST breakpoints were considered for the development of the Rapid FDC Acinetobacter baumannii NP test. When the CLSI breakpoints were considered (susceptible ≤ 4; intermediate: 8; resistant > 16 mg/L) [14] and setting intermediate and resistant isolates categorized as positive for the test, the performance of the test remained excellent, with only two ME (false positive, 2.7%, both presenting borderline MICs of 4 mg/L) and only a single VME (false negative, 5.0%, the same isolate that was found to be VME when interpreting using EUCAST), 95.0% of sensitivity (95% CI 76.4–99.1%), 97.3% specificity (95% CI 90.8–99.3%), 96.8% of accuracy, and 90.5% of precision.
The rapid FDC Acinetobacter baumannii NP test allows a quick assessment of FDC susceptibility/resistance when testing A. baumannii isolates. It nicely complements the rapid Cefiderocol NP test that had been recently developed for assessing the susceptibility/resistance to FDC in Enterobacterales [17]. Additionally, it is rather faster, cheap (± 1 USD per strain), and easier to perform if compared with the gold standard BMD which often results in questionable interpretation.
Following the urgent need for rapid and precise diagnostics in clinical settings, the rapid FDC Acinetobacter baumannii NP test was developed in order to provide a valuable method, which is easier and faster to interpret when compared with the recommended standard method. This rapid and inexpensive test can accurately categorize cefiderocol susceptibility/resistance of A. baumannii strains. The test showed remarkable sensitivity and specificity parameters; therefore, it may be suitable for implementation in any clinical microbiology laboratory.
Data Availability
All the data produced for this manuscript will be available upon request to the corresponding author.
References
World Health Organization (WHO) (2017) Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. WHO, Geneva, Switzerland https://apps.who.int/iris/bitstream/handle/10665/259462/9789241550178-eng.pdf?sequence=1&isAllowed=y. Accessed 10 August 2023
Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. https://doi.org/10.1111/j.1469-0691.2006.01456.x
Higgins PG, Dammhayn C, Hackel M, Seifert H (2010) Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238. https://doi.org/10.1093/jac/dkp428
World Health Organization (WHO) (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO, Geneva, Switzerland http://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf. Accessed 12 August 2023
Kollef M, Dupont H, Greenberg DE, Viale P, Echols R, Yamano Y, Nicolau DP (2023) Prospective role of cefiderocol in the management of carbapenem-resistant Acinetobacter baumannii infections: Review of the evidence. Int J Antimicrob Agents 62:106882. https://doi.org/10.1016/j.ijantimicag.2023.106882
Barnes MD, Kumar V, Bethel Moussa SH, O’Donnell J, Rutter JD, Good CE, Hujer KM, Hujer AM, Marshall SH, Kreiswirth BN, Richter SS, Rather PN, Jacobs MR, Papp-Wallace KM, van den Akker F, Bonomo R (2019) Targeting multidrug-resistant Acinetobacter spp.: Sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 10:e00159-19. https://doi.org/10.1128/mBio.00159-19
European Medicines Agency (2020) Fetcroja (cefiderocol) - Summary of product characteristics. April 2020. EMA, Amsterdam, The Netherlands https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf. Accessed 13 August 2023
U.S. Food and Drug Administration (2019) FETROJA (cefiderocol) - Highlights of prescribing information. November 2019. FDA, Silver Spring, Maryland, USA https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209445s000lbl.pdf. Accessed 13 August 2023
Poirel L, Sadek M, Nordmann P (2021) Contribution of PER-type and NDM-type β-lactamases to cefiderocol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 65:e0087721. https://doi.org/10.1128/AAC.00877-21
Nordmann P, Shields RK, Doi Y, Takemura M, Echols R, Matsunaga Y, Yamano Y (2022) Mechanisms of reduced susceptibility to cefiderocol among isolates from the CREDIBLE-CR and APEKS-NP clinical trials. Microb Drug Resist 28:398–407. https://doi.org/10.1089/mdr.2021.0180
Smoke SM, Brophy A, Reveron S, Iovleva A, Kline EG, Marano M, Miller LP, Shields RK (2023) Evolution and transmission of cefiderocol-resistant Acinetobacter baumannii during an outbreak in the burn intensive care unit. Clin Infect Dis 76:e1261–e1265. https://doi.org/10.1093/cid/ciac647
Morris CP, Bergman Y, Tekle T, Fissel JA, Tamma PD, Simner PJ (2020) Cefiderocol antimicrobial susceptibility testing against multidrug-resistant gram-negative Bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol 59:e01649-e1720. https://doi.org/10.1128/JCM.01649-20
U.S. Food and Drug Administration (2023) Cefiderocol injection - FDA Identified Breakpoints. January 2023. FDA, Silver Spring, Maryland, USA https://www.fda.gov/drugs/development-resources/cefiderocol-injection. Accessed 23 July 2023
Clinical and Laboratory Standards Institute (2023) Performance standards for antimicrobial susceptibility testing M100, 33rd edn. CLSI, Wayne, PA, USA
European Committee on Antimicrobial Susceptibility Testing (2023) Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0. January 2023. EUCAST, Växjö, Sweden https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf. Accessed 23 July 2023
European Committee on Antimicrobial Susceptibility Testing (2020) Guidance document on broth microdilution testing of cefiderocol. December 2020. EUCAST, Växjö, Sweden https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_201217.pdf. Accessed 19 July 2023
Nordmann P, Bouvier M, Poirel L, Sadek M (2022) Rapid cefiderocol NP test for detection of cefiderocol susceptibility/resistance in Enterobacterales. J Antimicrob Chemother 77:3456–3461. https://doi.org/10.1093/jac/dkac340
Nordmann P, Jayol A, Poirel L (2016) Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis 22:1038–1043. https://doi.org/10.3201/eid2206.151840
Funding
Open access funding provided by University of Fribourg This work was financed by the University of Fribourg, Switzerland, and by the National Reference Center of Emerging Antibiotic Resistance (NARA).
Author information
Authors and Affiliations
Contributions
The last author named is the lead and corresponding author (Patrice Nordmann). All the author contributions are described as follows: formal analysis: OHFR; investigation: AK, MB, and OHFR; methodology: MB and OHFR; validation: AK, LP, MB, OHFR, and PN; writing—original draft: OHFR; conceptualization: PN; supervision: PN and LP; writing—review and editing: LP and PN; and funding acquisition: PN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raro, O.H.F., Bouvier, M., Kerbol, A. et al. Rapid detection of cefiderocol susceptibility/resistance in Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 42, 1511–1518 (2023). https://doi.org/10.1007/s10096-023-04691-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04691-w