Abstract
This study aimed to report reference method antimicrobial susceptibility results for 24,937 recent (2019–2021) clinical isolates of Enterobacterales from 27 countries in Latin America, Eurasia, Africa/Middle East, and Asia with a focus on the investigational combination aztreonam–avibactam against metallo-β-lactamase (MBL) isolates. Antimicrobial susceptibility testing was performed by the CLSI broth microdilution methodology. Minimum inhibitory concentrations (MICs) were interpreted using the CLSI (2022) breakpoints for all agents except aztreonam–avibactam (provisional pharmacokinetic/pharmacodynamic susceptible breakpoint, ≤ 8 mg/L) and tigecycline (US-FDA). Molecular testing for β-lactamase genes was performed on isolates with meropenem MICs ≥ 2 mg/L, ceftazidime–avibactam MICs ≥ 16 mg/L, and/or aztreonam–avibactam MICs ≥ 16 mg/L, and 50% of isolates of Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella variicola, and Proteus mirabilis testing with ceftazidime and/or aztreonam MICs ≥ 2 mg/L. Aztreonam–avibactam inhibited 99.8% of all Enterobacterales at ≤ 8 mg/L (MIC90, 0.25 mg/L) and maintained activity against phenotypically resistant subsets of multidrug-resistant (MDR) (99.5% susceptible), extensively drug-resistant (XDR) (98.7%), and carbapenem-resistant Enterobacterales (CRE) (99.1%) isolates. At ≤ 8 mg/L, aztreonam–avibactam inhibited 100%, 99.6%, 99.6%, and 98.8% of KPC-, OXA-48-like-, ESBL-, and MBL-carrying isolates, respectively. MBL-positive isolates were most prevalent in India (20.5%), Guatemala (13.8%), and Jordan (13.2%). No differences in the activity of aztreonam–avibactam were observed across the global regions evaluated. At a concentration of ≤ 8 mg/L, aztreonam–avibactam inhibited almost all Enterobacterales collected from developing countries, including MBL-producing isolates. The widespread dissemination of MBLs among Enterobacterales highlights the unmet need for new agents such as aztreonam–avibactam for the treatment of CRE infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial resistance to antimicrobial agents is a well-documented threat to modern medicine. Among Gram-negative bacteria, the spread of acquired MBLs is particularly concerning, as they have the ability to hydrolyze all currently available β-lactam antibiotics, except for the monobactams and cefiderocol [1]. Recently approved β-lactam/β-lactamase inhibitor combinations, such as ceftazidime–avibactam, have proven activity against multidrug-resistant (MDR) bacterial infections [2]; however, ceftazidime–avibactam, imipenem–relebactam, and meropenem–vaborbactam are not active against metallo-β-lactamase (MBL)-harboring Gram-negative bacilli in vitro, leaving a gap in the current antimicrobial armamentarium and highlighting the need for new agents to treat infections caused by these pathogens [3]. Aztreonam–avibactam is in late development for use against infections caused by carbapenem-resistant Enterobacterales (CRE), including isolates carrying MBLs [4]. Aztreonam is refractory to hydrolysis by MBLs but is inactivated by ESBLs and other class A β-lactamases including KPCs and plasmid-mediated or stably derepressed chromosomally encoded class C (AmpC) β-lactamases. The β-lactamase inhibitor, avibactam, inhibits the activities of class A, C, and certain class D (including OXA-48-like) β-lactamases that are frequently co-carried with MBLs. As such, aztreonam–avibactam, if approved, would represent an attractive therapeutic option for use against MBL-harboring Enterobacterales regardless of whether or not they co-carried serine β-lactamase(s).
The Antimicrobial Testing Leadership and Surveillance (ATLAS) Global Surveillance Program offers a means to annually monitor the in vitro activity of antimicrobials, including aztreonam–avibactam, against clinical isolates of Gram-negative bacilli collected worldwide. Isolates associated with bloodstream, intra-abdominal, respiratory tract, skin and soft tissue, and urinary tract infections are included in the program. The current study focused on the in vitro activity of aztreonam–avibactam and comparators against Enterobacterales collected in 27 countries in Latin America, Eurasia, the Africa/Middle East region, and Asia in 2019–2021 for which current, robust, and country-specific in vitro aztreonam–avibactam data is not widely available.
Materials and methods
Bacterial isolates
Isolates of Enterobacterales (n = 24,937) included in the present study were collected as a part of the ATLAS Global Surveillance Program from 27 countries worldwide between 2019 and 2021. For analysis, countries were grouped into four geographic regions as follows: the Africa/Middle East region included Cameroon, Ivory Coast, Jordan, Kuwait, Morocco, Nigeria, Qatar, Saudi Arabia, and South Africa; Asia included Hong Kong, India, Malaysia, the Philippines, Taiwan, and Thailand; Eurasia included Russia and Turkey; and Latin America included Argentina, Brazil, Chile, Colombia, Costa Rica, Dominican Republic, Guatemala, Mexico, Panama, and Venezuela (Supplemental Table S1). Medical centers in each country participated in the ATLAS program in all 3 years with the exception of Cameroon and Ivory Coast which participated only in 2020 and 2021, and Russia, from which isolates were only available in 2019. Organisms in the present study were isolated from the bloodstream (n = 5921), intra-abdominal (n = 3263), lower respiratory tract (n = 4907), skin and soft tissue (n = 4400), urinary tract (n = 6408), and other unspecified (n = 38) infection sources. Isolates collected from ICU patients accounted for 26.7% (6632/24,937) of the total isolates. Bacterial species identities were confirmed by IHMA (Schaumburg, IL, USA) using the MALDI-TOF mass spectrometry (Bruker Daltonics, Billerica, MA, USA).
The ATLAS program requests each participating medical center laboratory to collect annually defined quotas of isolates of selected bacterial species from patients with bloodstream infections, intra-abdominal infections, lower respiratory tract infections, skin and soft tissue infections, and urinary tract infections. It was limited to one isolate per species per patient. All isolates were determined to be clinically significant by participating laboratory algorithms and were collected irrespective of antimicrobial susceptibility profile. The ATLAS program (https://atlas-surveillance.com) is not intended to evaluate the geographic prevalence of bacteria causing specific infection types. As with all large-scale antimicrobial resistance surveillance networks, ATLAS does experience some variation in the number of participating centers in each study year, center constancy between study years, and distribution of centers in each region.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed in a central laboratory (IHMA) using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method [5]. Avibactam was tested at a fixed concentration of 4 mg/L. Minimum inhibitory concentrations (MICs) were interpreted using the 2022 CLSI [6] breakpoints (interpretation via 2022 EUCAST criteria [7] is provided in Supplemental Table S2). United States Food and Drug Administration (FDA) MIC interpretative breakpoints were used for tigecycline [8] in place of CLSI breakpoints which do not exist. Aztreonam–avibactam MICs were interpreted using a previously established provisional pharmacokinetic/pharmacodynamic susceptible breakpoint of ≤ 8 mg/L [9,10,11].
Isolates were identified as CRE on the basis of imipenem or meropenem MIC values ≥ 4 mg/L, with the exception that imipenem was excluded from CRE screening of Morganellaceae due to its intrinsically elevated MIC values in that family. Isolates were categorized as MDR or XDR according to criteria published in 2012 by the joint European and United States Centers for Disease Control [12], which define an MDR isolate as one that is nonsusceptible to ≥ 1 agent in ≥ 3 antimicrobial classes and an XDR isolate as one that is susceptible to ≤ 2 classes from the agents tested. The antimicrobial classes and specific antimicrobial agent representatives in this analysis were cephalosporins (ceftazidime, cefepime), cephalosporins combined with β-lactamase inhibitor (ceftazidime–avibactam), carbapenems (imipenem, meropenem), fluoroquinolones (levofloxacin), aminoglycosides (gentamicin, amikacin), monobactams (aztreonam), polymyxins (colistin), and glycylcyclines (tigecycline).
Molecular analysis
Isolates of Enterobacterales that tested with a meropenem MIC of ≥ 2 mg/L (not susceptible by the CLSI breakpoint), a ceftazidime–avibactam MIC of ≥ 16 mg/L (not susceptible by the CLSI breakpoint), and/or an aztreonam–avibactam MIC of ≥ 16 mg/L were screened for the presence of genes encoding serine carbapenemases (KPC, OXA-48-like, and GES), MBLs (NDM, IMP, VIM, SPM, and GIM), ESBLs (SHV, TEM, CTX-M-1 group, CTX-M-2 group, CTX-M-8 group, CTX-M-9 group, CTX-M-25 group, VEB, PER, and GES), and acquired AmpC β-lactamases (ACC, ACT, CMY, DHA, FOX, MIR, and MOX) using multiplex PCR assays, followed by amplification and sequencing of the full-length genes and comparison to publicly available databases, as previously described [13]. Additionally, 50% of meropenem-susceptible (MIC ≤ 1 mg/L) isolates of Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella variicola, and Proteus mirabilis testing with ceftazidime or aztreonam MICs ≥ 2 mg/L (CLSI ESBL screening criteria) [6] were interrogated for β-lactamase genes following the same procedure. The acquired β-lactamase carriage of isolates testing with aztreonam–avibactam MIC values ≥ 16 mg/L is provided in the “Supplemental Table S3.”
Results
Table 1 summarizes the in vitro susceptibility testing data for 12 antimicrobial agents against all 24,937 Enterobacterales isolates, collected from 2019 to 2021, from the 27 countries. The growth of 99.8% of isolates from the full collection was inhibited by aztreonam–avibactam at a concentration of ≤ 8 mg/L, the provisional pharmacokinetic/pharmacodynamic susceptible breakpoint [9,10,11], and the MIC90 value was 0.25 mg/L. Only 61 of the 24,937 (0.2%) isolates tested with an aztreonam–avibactam MIC > 8 mg/L, including 38 E. coli, seven K. pneumoniae, five P. mirabilis, three Providencia rettgeri, and eight members of other species of Enterobacterales. Tigecycline (96.4% susceptible), ceftazidime–avibactam (93.1%), and amikacin (91.3%) also inhibited > 90% of all isolates at their respective susceptible breakpoints: 86.9% of all isolates were meropenem-susceptible, and aztreonam (59.9% susceptible), cefepime (60.6%), and ceftazidime (59.6%) showed similar activities. Only 54.8% of all Enterobacterales isolates were levofloxacin-susceptible.
Aztreonam–avibactam displayed consistently potent activity against Enterobacterales across all geographic regions analyzed. At a concentration of ≤ 8 mg/L, aztreonam–avibactam was inhibited from 99.4% (Asia) to > 99.9% (Latin America) of isolates in each geographic region (Table 1). Only seven of 5245 isolates from countries in the Africa/Middle East region displayed an aztreonam–avibactam MIC value > 8 mg/L: two from Nigeria, two from Morocco, and one each from Ivory Coast, Saudi Arabia, and Qatar. These organisms included two E. coli, and one each of K. pneumoniae, Morganella morganii, P. mirabilis, Enterobacter asburiae, and unspeciated Enterobacter sp. Among countries in Asia, 45 of 8172 isolates had an aztreonam–avibactam MIC value > 8 mg/L, including E. coli (n = 31), K. pneumoniae (n = 5), P. mirabilis (n = 3), Providencia rettgeri (n = 3), Citrobacter koseri (n = 1), Providencia stuartii (n = 1), and unspeciated Providencia sp. (n = 1). The great majority of these isolates (41/45, 91.1%) were collected in India, with two originating from Thailand and one each from the Philippines and Taiwan. Only six of 2003 isolates collected in Eurasian countries tested with MIC values > 8 mg/L: five originated from Turkey and one from Russia, consisting of four E. coli and one each of Citrobacter freundii and K. pneumoniae. Just three of the 9517 isolates collected in Latin American countries, one E. coli, one Klebsiella aerogenes isolate originating from Colombia, and one P. mirabilis isolate from Brazil, displayed aztreonam–avibactam MIC values > 8 mg/L.
For the full collection, 12,192 (48.9%), 2974 (11.9%), and 3289 (13.2%) of isolates were identified as MDR, XDR, and CRE, respectively. Aztreonam–avibactam retained in vitro activity against these phenotypic subsets of Enterobacterales isolates, with 99.5% of MDR, 98.7% of XDR, and 99.1% of CRE isolates inhibited at ≤ 8 mg/L (Table 1). No comparator agent inhibited > 90% of MDR, XDR, or CRE isolates other than tigecycline which inhibited 93.4% of MDR and 94.4% of CRE isolates.
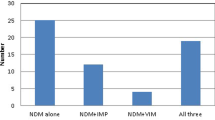
Overall, 7446 Enterobacterales isolates were eligible for β-lactamase gene screening based upon the qualifying criteria. Among these, 1610 were identified as carrying one or more MBLs, including 1544 isolates carrying NDM, 27 carrying VIM, 28 carrying IMP, six co-carrying NDM and VIM, and five co-carrying NDM and IMP. Figure 1 provides the taxonomic distribution of MBL-producing isolates by enzyme family. K. pneumoniae harbored NDM and VIM more frequently (60.1% and 45.4% of the NDM and VIM carriers, respectively) than other species of Enterobacterales, whereas IMP was found in an equal number of K. pneumoniae and Enterobacter spp. isolates (each genus/species accounted for 24.2% of all IMP carriers). Aztreonam–avibactam at a concentration of ≤ 8 mg/L (MIC90, 2 mg/L) inhibited 98.8% of all MBL producers, including 100% of VIM and IMP carriers and 98.7% of NDM carriers. All comparator antimicrobial agents were inactive against MBL producers with the exception of tigecycline which inhibited 93.8% of isolates (Table 1). It should be noted that the meropenem-nonsusceptibility threshold that triggered screening (MIC of ≥ 2 mg/L) could potentially miss some carbapenemase-carrying organisms for which the carbapenemase is weakly expressed [14].
In addition, among the molecularly characterized isolates, 705 were identified that harbored KPC (excluding those that co-carried an MBL), 831 carried an OXA-48-like enzyme (excluding those that co-carried an MBL or KPC), and 3605 harbored an ESBL (excluding those that co-carried a carbapenemase). Aztreonam–avibactam at a concentration of ≤ 8 mg/L inhibited 100%, 99.6%, and 99.6% of KPC-, OXA-48-like-, and ESBL-carrying isolates, respectively (Table 1). Among comparator agents, > 98% of KPC-, OXA-48-like-, and ESBL-carrying isolates were ceftazidime–avibactam-susceptible and ≥ 95% were tigecycline-susceptible.
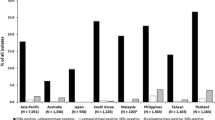
Given the potential for aztreonam–avibactam to treat patients infected with MBL-producing Enterobacterales isolates, further analyses were performed on the subset of 1610 isolates that carried MBLs, the great majority of which harbored genes for NDM-type enzymes (1555/1610, 96.6% carried NDM, either as the sole MBL or with additional MBLs). Figure 2 summarizes the percentage of MBL producers identified among the total number of isolates collected in each of the evaluated countries. MBL-positive isolates were most common in India (20.5%), Guatemala (13.8%), and Jordan (13.2%), three geographically diffuse countries, but were rare (≤ 2%) in Cameroon, Chile, Costa Rica, Dominican Republic, Ivory Coast, Panama, Saudi Arabia, and Turkey, and completely absent in Hong Kong. Aztreonam–avibactam at a concentration of ≤ 8 mg/L inhibited 100%, 100%, 98.5%, and 97.9%, of MBL-positive Enterobacterales isolates from the Africa/Middle Eastern, Latin American, Eurasian, and Asian regions, respectively (Table 1). Figure 3 provides country-specific data for both aztreonam–avibactam and aztreonam against MBL-harboring isolates and shows that at a concentration of ≤ 8 mg/L, aztreonam–avibactam inhibited the growth of all MBL-harboring isolates from all countries but two: India, where aztreonam–avibactam at a concentration of ≤ 8 mg/L inhibited 97.4% of isolates, and Russia, where 97.6% of isolates were inhibited. These results are in contrast to aztreonam alone, against which the susceptibility of MBL-harboring isolates ranged from 66.7% in Chile to 0% in Costa Rica, Dominican Republic, and Jordan.
Discussion
Aztreonam–avibactam demonstrated potent activity against a recent set of Enterobacterales originating from twenty-seven countries in Latin America, Eurasia, Africa/Middle East, and Asia. Importantly, the in vitro activity of aztreonam–avibactam was maintained against MBL producers, a group that currently presents clinicians with limited treatment options. The results presented here on contemporary isolates (2019–2021) confirm earlier reports on global collections focused on aztreonam–avibactam susceptibility. Karlowsky et al. described the activity of aztreonam–avibactam and comparators against a large dataset including Enterobacterales (n = 51,352) and Pseudomonas aeruginosa (n = 11,842) collected from 40 countries from 2012 to 2015 [15]. That study reported that > 99.9% of all Enterobacterales isolates and 99.8% of meropenem-nonsusceptible Enterobacterales (n = 1498) were inhibited by aztreonam–avibactam at a concentration of ≤ 8 mg/L. Additionally, all 267 Enterobacterales isolates positive for MBL genes (NDM, VIM, IMP) demonstrated aztreonam–avibactam MICs of ≤ 8 mg/L. An earlier study by Biedenbach et al. evaluated aztreonam–avibactam and comparator antimicrobial agents against 28,501 unique clinical isolates of Enterobacterales, P. aeruginosa, and Acinetobacter baumannii collected in 39 countries in 2012–2013 [16]. In that study, aztreonam–avibactam inhibited 99.9% of all Enterobacterales, and > 99% of the MBL-producing isolates of Enterobacterales at ≤ 4 mg/L. Sader et al. recently reported on a set of 24,924 Enterobacterales collected in Europe, Asia, and Latin America as a part of the SENTRY program from both developed and developing countries in 2019–2021 [17]. They demonstrated that 99.6% of CRE were inhibited at an aztreonam–avibactam concentration of ≤ 8 mg/L, including 99.9% of isolates carrying a carbapenemase gene. Similarly, Rossolini analyzed Enterobacterales isolates collected globally (54 countries) in 2019 and showed that aztreonam–avibactam possessed potent antimicrobial activity against all Enterobacterales, with 99.9% of isolates, including > 99% of those carrying MBLs, inhibited at a concentration of ≤ 8 mg/L [18].
In the present study, only 61 of 24,937 (0.2%) isolates were tested with an aztreonam–avibactam MIC > 8 mg/L, including 38 E. coli, seven K. pneumoniae, five P. mirabilis, three Providencia rettgeri, and eight members of other species of Enterobacterales. In E. coli, elevated aztreonam–avibactam MIC values have been associated with four amino acid insertions in a region of the penicillin-binding protein 3 (PBP3) adjacent to the β-lactam binding pocket [19,20,21]. Recently, Sadek et al. demonstrated that although the previously described PBP3 insertions contribute to decreased aztreonam–avibactam susceptibility, they cannot be considered the unique basis of resistance as the presence of a transmissible AmpC-type β-lactamase, CMY (particularly the CMY-42 variant), and also contributes to elevated MICs [22]. Although a thorough investigation of mechanisms of resistance to aztreonam–avibactam was beyond the scope of this report, it is noteworthy that 28 of the 38 (71.1%) E. coli isolates with aztreonam–avibactam MIC values > 8 mg/L were found to harbor CMY (Supplemental Table S3). Twenty of those carried CMY-42, six carried CMY-145, and one each carried CMY-141 and CMY-146. CMY-141, CMY-145, and CMY-146 each differ from CMY-42 by a single amino acid substitution. Additionally, recent evidence suggests that an elevation of CMY gene expression levels, as would be expected with an increase in the copy number of the plasmid carrying the gene, also contributes to reduced aztreonam–avibactam susceptibility [23].
In conclusion, the current data on recent clinical isolates collected worldwide confirms the potent activity of aztreonam–avibactam against Enterobacterales, including isolates that carry MBLs. As MBL-producing Enterobacterales become more prevalent in healthcare systems across regions and therapeutic options to treat serious infections caused by MBL carriers are presently extremely limited, aztreonam–avibactam represents a potentially valuable option for the treatment of infections caused by MBL-producing MDR and XDR Enterobacterales.
Data availability
The data generated and/or analyzed for the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Boyd SE, Livermore DM, Hooper DC, Hope WW (2020) Metallo-β-lactamases: structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob Agents Chemother 64:e00397-e420. https://doi.org/10.1128/AAC.00397-20
Lagacé-Wiens P, Walkty A, Karlowsky JA (2014) Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. https://doi.org/10.2147/CE.S40698
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL et al (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Mauri C, Maraolo AE, Di Bella S, Luzzaro F, Principe L (2021) The revival of aztreonam in combination with avibactam against metallo-β-lactamase-producing Gram-negatives: a systematic review of in vitro studies and clinical cases. Antibiotics (Basel) 20:1012. https://doi.org/10.3390/antibiotics10081012
Clinical and Laboratory Standards Institute (2018) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Eleventh Edition: Approved Standard M07-A11. CLSI, Wayne, PA, USA
Clinical and Laboratory Standards Institute (2022) Performance standards for antimicrobial susceptibility testing. M100. Thirty-Second Edition. CLSI, Wayne, PA, USA
EUCAST (2022) v12.0. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. January 2022. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden
https://www.fda.gov/drugs/development-resources/tigecycline-injection-products. Accessed 13 March 2023
Cornely OA, Cisneros JM, Torre-Cisneros J, Rodríguez-Hernández MJ, Tallón-Aguilar L, Calbo E et al (2020) Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: results from the REJUVENATE study. J Antimicrob Chemother 75:618–627. https://doi.org/10.1093/jac/dkz497
Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S et al (2015) Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. https://doi.org/10.1093/jac/dkv132
Sader HS, Carvalhaes CG, Arends SJR, Castanheira M, Mendes RE (2021) Aztreonam/avibactam activity against clinical isolates of Enterobacterales collected in Europe, Asia and Latin America in 2019. J Antimicrob Chemother 76:659–666. https://doi.org/10.1093/jac/dkaa504
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Lob SH, Kazmierczak KM, Badal RE, Hackel MA, Bouchillon SK, Biedenbach DJ, Sahm DF (2015) Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART program, 2009 to 2013. Antimicrob Agents Chemother 59:3606–3610. https://doi.org/10.1128/AAC.05186-14
Fattouh R, Tijet N, McGeer A, Poutanen SM, Melano RG, Patel SN (2015) What is the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence pettings? Antimicrob Agents Chemother 60:1556–1559. https://doi.org/10.1128/AAC.02304-15
Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA (2017) In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 61:e00472-e517. https://doi.org/10.1128/AAC.00472-17
Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA (2015) In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 59:4239–4248. https://doi.org/10.1128/AAC.00206-15
Sader HS, Castanheira M, Kimbrough JH, Kantro V, Mendes RE (2023) Aztreonam/avibactam activity against a large collection of carbapenem-resistant Enterobacterales (CRE) collected in hospitals from Europe, Asia and Latin America (2019–21). JAC Antimicrob Resist 5(2):dlad032. https://doi.org/10.1093/jacamr/dlad032.
Rossolini GM, Stone G, Kantecki M, Arhin FF (2022) In vitro activity of aztreonam/avibactam against isolates of Enterobacterales collected globally from ATLAS in 2019. J Glob Antimicrob Resist 30:214–221. https://doi.org/10.1016/j.jgar.2022.06.018
Alm RA, Johnstone MR, Lahiri SD (2015) Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother 70:1420–1428. https://doi.org/10.1093/jac/dku568
Mendes RE, Doyle TB, Streit JM, Arhin FF, Sader HS, Castanheira M (2021) Investigation of mechanisms responsible for decreased susceptibility of aztreonam/avibactam activity in clinical isolates of Enterobacterales collected in Europe, Asia and Latin America in 2019. J Antimicrob Chemother 76:2833–2838. https://doi.org/10.1093/jac/dkab279
Estabrook M, Kazmierczak KM, Wise M, Arhin FF, Stone GG, Sahm DF (2021) Molecular characterization of clinical isolates of Enterobacterales with elevated MIC values for aztreonam-avibactam from the INFORM global surveillance study, 2012–2017. J Glob Antimicrob Resist 24:316–320. https://doi.org/10.1016/j.jgar.2021.01.010
Sadek M, Juhas M, Poirel L, Nordmann P (2020) Genetic features leading to reduced susceptibility to aztreonam-avibactam among metallo-β-lactamase-producing Escherichia coli isolates. Antimicrob Agents Chemother 64:e01659-e1720. https://doi.org/10.1128/AAC.01659-20
Ma K, Feng Y, McNally A, Zong Z (2020) Struggle to survive: the choir of target alteration, hydrolyzing enzyme, and plasmid expression as a novel aztreonam-avibactam resistance mechanism. mSystems 5:e00821-20
Acknowledgements
The authors thank Michal Kantecki and Francis Arhin for valuable comments on an earlier version. Additionally, all ATLAS participating investigators are thankful for their contributions to the program.
Funding
This study was sponsored by Pfizer, which included compensation for services related to preparing this manuscript.
Author information
Authors and Affiliations
Contributions
The authors listed, wrote, edited, and/or reviewed this manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required because all isolates received into the study followed multiple subcultures and were completely de-identified. The secondary research use of de-identified isolates is considered exempt research according to the Regulations for the Protection of Human Subjects in Research of the U.S. Department of Health and Human Services, Office for Human Research Protections (45 CFR 46).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MGW and DFS are employees of IHMA, who were paid consultants to Pfizer in connection with the development of this manuscript. JAK is a consultant to IHMA. MGW, DFS, and JAK do not have a personal financial interest in the sponsor of this work (Pfizer, Inc.). NM and SK are employees of Pfizer.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wise, M.G., Karlowsky, J.A., Mohamed, N. et al. In vitro activity of aztreonam–avibactam against Enterobacterales isolates collected in Latin America, Africa/Middle East, Asia, and Eurasia for the ATLAS Global Surveillance Program in 2019–2021. Eur J Clin Microbiol Infect Dis 42, 1135–1143 (2023). https://doi.org/10.1007/s10096-023-04645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04645-2