Abstract
The immunogenicity and safety of vaccines against coronavirus disease 2019 (COVID-19) remain unknown in patients with a history of pulmonary tuberculosis (OPTB). Therefore, the safety and effectiveness of inactivated vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were assessed in patients with a history of PTB. The study cohort included 106 healthy controls and 93 adult patients with OPTB who received a two-dose vaccination. The study period was 21 to 105 days. Concentrations of antibodies (Abs) against receptor-binding domain (RBD) IgG and SARS-CoV-2 neutralizing Abs (NAbs) were measured, in addition to the frequencies of SARS-CoV-2-specific B and a portion T cells. The incidence of adverse events was similar between the OPTB patients and healthy controls. No severe adverse events occurred. Concentrations of Abs against RBD-IgG and CoV-2 neutralizing Abs in addition to the frequencies of RBD-specific memory B cells proportions were lower in OPTB patients than the healthy controls (all, p < 0.05), while the frequencies of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4+) cells were higher (p = 0.023). There was no obvious correlation between age and blood concentrations of Abs against RBD-IgG and CoV-2 neutralizing Abs, while immune responses were similar in the fibrosis and calcification groups. The period of time following full-course vaccination and lymphocyte counts were associated to anti-RBD-IgG responses. Inactivated COVID-19 vaccinations were well tolerated in OPTB patients, although immunogenicity was limited in this population. This study has been registered at ClinicalTrials.gov (NCT05043246).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and the ensuing coronavirus disease 2019 (COVID-19) pandemic continues to pose severe impacts to human health worldwide and is especially detrimental to patients with a history of pulmonary tuberculosis (OPTB), as demonstrated by the particularly high morbidity rate [1,2,3,4]. Vaccination is crucial to prevent SARS-CoV-2 infection and is especially useful to reduce the incidences of severe symptoms and death [5,6,7]. B cells are crucial for vaccine-mediated immunity and memory B cells (MBCs), in particular, are necessary for the generation of antibodies (Abs). T cells, meanwhile, have unique receptors to identify specific pathogens [8,9,10]. Regulatory T cells (Tregs), a subpopulation of T cells that maintain immune homeostasis, have been linked to the regulation of PTB-specific immune responses [11]. Previous studies have reported decreased proportions of naïve B cells and MBCs in PTB patients with increased proportions of atypical B cells [12,13,14]. In addition, the ability of vaccines to induce an immune response against SARS-CoV-2 in OPTB patients remains unknown. Therefore, the aim of the present study was to assess the safety and effectiveness of inactivated vaccines against SARS-CoV-2 in patients with a history of PTB.
Materials and methods
Recruitment and clinical sample collection
The study cohort consisted of 106 healthy adults and 93 adults with a history of PTB who were recruited from the Second Affiliated Hospital of Chongqing Medical University between July 15, 2021, and December 3, 2021. The diagnosis of OPTB was based on established guidelines. The inclusion criteria were (i) a full course of vaccination with BBIBP-CorV (China National Pharmaceutical Group Co., Ltd., Beijing, China) or CoronaVac (Sinovac Biotech Ltd., Beijing, China), (ii) age ≥ 18 years, (iii) no previous SARS‐CoV‐2 infection, and (iv) normal immune function. Pregnant women were excluded from the study.
Peripheral blood samples from participants were arbitrarily taken one or more at intervals of at least 21 days (21–105 days) following the whole course of vaccination in order to test for Abs against RBD-IgG, SARS-CoV-2 neutralizing antibodies, and RBD-specific B cells and a portion of T cells. In this study, 21–45 days after immunization was defined as “1 month,” 46–75 days as “2 months,” and 76–105 days as “3 months.”
The OPTB patients were assigned to the calcification group (n = 54, calcification of nodules and lymph nodes as determined by low-dose spiral computed tomography [CT]) or the fibrosis group (n = 51, presence of fibrosis as determined by low-dose spiral CT). The two groups were then divided into four subgroups based on age (≥ 55 and < 55 years).
Monitoring of adverse events (AEs)
AEs were assessed using a questionnaire at 7–30 days after vaccination and categorized in accordance with the guidelines established by the China Medical and Drug Administration (2019 edition).
Detection of Abs against SARS-CoV-2
Serum levels of Abs against S-RBD-IgG were measured with an automatic chemiluminescence analyzer (MAGLUMI 2000; Snibe Co., Ltd., Shenzhen, China). The critical value of anti-RBD-IgG was set at 1 AU/mL, while the critical value of neutralizing Abs was 0.15 µg/mL in accordance with the manufacturer’s instructions. A positive Ab result indicates that the test results were greater than the associated critical value, while a negative Ab result indicates that the test results were either less than or equal to the crucial value.
Detection of SARS-CoV-2-specific B cells by flow cytometry
Stained peripheral blood mononuclear cells were assessed by flow cytometry (Beckman Coulter, Inc., Brea, CA, USA). The antigen probe was a combination of the biotinylated SARS-CoV-2 Spike RBD protein (40,592-V08H2-B; Sino Biological, Inc., Beijing, China) and streptavidin-BV421 (405,225; BioLegend, San Diego, CA, USA) at a molar ratio of 4:1. The peripheral blood mononuclear cells were isolated by density gradient centrifugation using Histopaque® density gradient media (Sigma-Aldrich Corporation, St. Louis, MO, USA). After staining with buffered saline solution with 2% fetal bovine serum, the cells were washed and then probed with Abs (all purchased from BioLegend) against IgG Fc, IgM, cluster of differentiation (CD) 3, CD19, CD21, and CD27 for 30 min at 4 °C in the dark. The resulting data were analyzed with FlowJo™ software (version 10.0.7; BD Biosciences, San Jose, CA, USA).
Detection of Tregs by flow cytometry
Similarly, Tregs were analyzed by flow cytometry with fluorescent Abs (all purchased from BioLegend and diluted to 1:50) against CD233 (lymphocyte activation gene 3, LAG-3), CD152 (cytotoxic T-lymphocyte-associated protein 4, CTLA-4), CD279 (programmed cell death protein 1, PD-1), CD39, CD4, CD25, CD45RA, CD3, CD127 (interleukin-7 receptor subunit alpha, IL7R-α), and CD134 (tumor necrosis factor receptor superfamily member 4, OX40).
Cells positive for interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) were detected with FluoroSpot test kits (Mabtech AB, Nacka Strand, Sweden). Briefly, the plates were pre-coated with monoclonal Abs against IFN-γ and TNF-α (1-D1K and MT25C5, respectively), washed three times with sterile phosphate-buffered saline (PBS; 200 μL/well), and incubated with 10% FBS (100 μL/well; ExCell Biotech Co., Ltd., Shanghai, China) at room temperature for 30 min. After removal of the medium, unstimulated cells as a control group (100 μL/well) and cells stimulated with the SARS-CoV-2 spike protein (1 μL/well; Sino Biological, Inc.) and Abs against CD28 (0.1 μL/well; dilution, 1:1000; Life Technologies, Carlsbad, CA, USA) were added to the appropriate wells. As positive controls, cells were stimulated with Abs (dilution, 1:50; BioLegend) against CD28 and CD3 (0.1 μL/well). The plates were incubated at 37 °C for 24 h under an atmosphere of 5% CO2/95% air. All experiments were performed in duplicate. After washing the plates five times with PBS, monoclonal Abs (Mabtech AB) against IFN-γ (dilution, 1:200) and TNF-α (dilution, 1:250) were added to the wells and the plate was incubated for 2 h at room temperature, then washed five times again with PBS, followed by the addition of secondary Abs (BMA-490 and SA-550; dilution, 1:200; Mabtech AB). Finally the results were interpreted with an automated FluoroSpot reader (Elispot Reader; Autoimmun Diagnostika GmbH, Strassberg, Germany).
Statistical analysis
The chi-square and Fisher’s exact tests were used for comparisons of categorical variables, while the Student t-test and Mann–Whitney U test were used for comparisons of continuous variables with normal and non-normal distributions, respectively. Comparisons of three or more groups were conducted with the Kruskal–Wallis test followed by the Bonferroni post hoc test. Factors that significantly impacted Ab titers were identified by univariate and multivariate ordinal linear regression analyses. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corporation, Armonk, NY, USA) and figures were generated with GraphPad Prism software version 9.2.0 (GraphPad Software, Inc., San Diego, CA, USA). A probability (p) value < 0.05 was considered statistically significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Results
The characteristics of the study participants after full-course vaccination are shown in Table 1. There were no significant differences in the median age, median body mass index (BMI), and proportions of males between the OPTB patients and healthy controls. The median period for vaccine immunogenicity after inoculation of the OPTB patients and healthy controls was 56 (range, 21–103) days and 57 (range, 21–105) days, respectively. White blood cell, platelet, hemoglobin, lymphocyte, total protein, aspartate transaminase, alanine aminotransferase, creatinine, and uric acid levels were similar between the OPTB patients and controls, while albumin levels were lower in OPTB patients. The characteristics of the study participants at 1, 2, and 3 months after full-course vaccination are shown in Supplementary Table 1.
AEs associated with vaccination against COVID-19 are shown in Table 2. The overall incidence of AEs within 7 days after vaccination was similar between the OPTB patients and healthy controls (10.7% vs. 11.3%, respectively, p = 0.899). Injection site pain was the most common local AE, occurring in 3.2% of OPTB patients and 4.7% of healthy controls. All AEs were uncommon, affecting < 5% of the OPTB patients and healthy controls, mild (grades 1 and 2), and resolved spontaneously within 7 days. There were no severe AEs (grade 3/4), such as severe thromboembolism and myocarditis. After 30 days, two of the healthy controls reported mild AEs (one case each of injection site pain and abdominal pain).
Humoral immune response to inactivated SARS-CoV-2 vaccines in OPTB
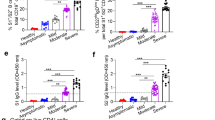
As compared to healthy controls, the seropositivity rates of OPTB patients were significantly decreased for Abs against RBD-IgG (70.9% vs. 90.6%, respectively, p = 0.010) and SARS-CoV-2 neutralizing Abs (61.3% vs. 84.9%, respectively, p < 0.001). Likewise, concentrations of Abs against RBD IgG were significantly lower for PTB patients than the healthy controls (median [IQR]: 2.15 [0.85–4.71] vs. 4.19 [1.76–7.01], respectively, p = 0.001), as well as concentrations of CoV-2 neutralizing Abs (median [IQR]: 0.195 [0.117–0.308] vs. 0.284 [0.175–0.404], respectively, p < 0.001) (Fig. 1a–d).
a–d Antibody responses to inactivated SARS-CoV-2 vaccines in OPTB. The seropositivity rates (b, d) and concentrations of (a, c) Abs against RBD-IgG and SARS-CoV-2 neutralizing Abs in all OPTB patients and healthy controls, respectively. Chi-square test, Fisher’s exact test, and Mann–Whitney U test (interquartile range, IQR) were used for two-group comparison (healthy controls and OPTB patients). The limit of detection range of concentrations of anti-RBD-IgG{0.375–1000AU/mL}, the limit of detection range of concentrations of CoV-2 NAb{0.05–30ug/mL}. The p-values represented in this figure are all adjusted p-values. e RBD+-specific B cells responses to inactivated SARS-CoV-2 vaccines in OPTB. The frequencies of e B cells, RBD-specific B cells, RBD+ resting MBCs, RBD+ activated MBCs, RBD+ atypical MBCs, RBD+ intermediate MBCs, RBD-specific memory B cells (MBCs), RBD-specific MBCs IgG+, and RBD-specific MBCs IgM+ in all OPTB patients and healthy controls. Mann–Whitney U test (interquartile range, IQR) was used for two-group comparison (healthy controls and OPTB patients). The p-values represented in this figure are all adjusted p-values. MBC, memory B cell
The frequencies of RBD+ atypical MBCs were greater in all OPTB patients than healthy controls, while the frequencies of RBD-specific MBCs and RBD+ resting MBCs were lower (median [IQR]: 29.6 [21.23–46.30] vs. 22.0 [16.95–29.10], p < 0.001, 35.0 [27.15–40.03] vs. 41.70 [33.95–52.35], p = 0.006, and 12.25 [4.04–21.125] vs. 21.10 [15.40–26.10], p < 0.001, respectively) (Fig. 1e).
Humoral immune response to inactivated SARS-CoV-2 vaccines at 1, 2, and 3 months
As compared to the healthy controls, the OPTB patients had lower seropositivity rates at 1 month for Abs against RBD-IgG (84.1% vs. 97.8%, respectively, p = 0.028) and SARS-CoV-2 neutralizing Abs (70.5% vs. 97.8%, respectively p < 0.001), in addition to concentrations Abs against RBD-IgG (median [IQR], 2.71 [1.71–5.51] vs. 5.58 [4.21–11.01], p < 0.001) and SARS-CoV-2 neutralizing Abs(median [IQR], 0.21 [0.11–0.39] vs. 0.36 [0.26–0.47], p = 0.001) (Fig. 2a–d). The seropositivity rates and concentrations of both Abs were somewhat lower in OPTB patients than healthy controls at 2 and 3 months, although these differences were not statistically significant (Supplementary Fig. 1).
a–d Antibody responses to inactivated SARS-CoV-2 vaccines at 1 month. The seropositivity rates (b, d) and concentrations of (a, c) Abs against RBD-IgG and SARS-CoV-2 neutralizing Abs in OPTB patients and healthy controls at 1 month, respectively. Chi-square test, Fisher’s exact test, and Mann–Whitney U test (interquartile range, IQR) were used for two-group comparison (healthy controls and OPTB patients). The limit of detection range of concentrations of anti-RBD-IgG{0.375–1000AU/mL}, the limit of detection range of concentrations of CoV-2 NAb{0.05–30ug/mL}. The p-values represented in this figure are all adjusted p-values. e–h RBD+-specific B cells responses to inactivated SARS-CoV-2 vaccines at 1, 2, and 3 months. The frequencies of e RBD+ resting MBCs, f RBD+ atypical MBCs, g RBD-specific memory B cells (MBCs), and h RBD-specific MBCs IgM+ in OPTB patients and healthy controls at 1, 2, and 3 months. Student’s t-test (confidence interval, CI) and Mann–Whitney U test (interquartile range, IQR) were used for two-group comparison (healthy controls and OPTB patients). The p-values represented in this figure are all adjusted p-values. MBC, memory B cell
At 1 month, the frequencies of RBD-specific MBCs and RBD+ resting MBCs were lower in OPTB patients than healthy controls (median [IQR], 35.0 [24.90–51.30] vs. 43.5 [35.60–54.23], p = 0.024, and 11.95 [1.78–19.70] vs. 20.10 [14.70–25.95], p = 0.002, respectively), while the frequencies of RBD+ atypical MBCs were higher (median [IQR], 33.35 [18.33–59.03] vs. 23.10 [17.93–31.35], respectively, p = 0.020). At 3 months, the frequencies of RBD+ resting MBCs were lower and the frequencies of RBD-specific MBCs IgM+, and RBD+ atypical MBCs were greater in OPTB patients than healthy controls (13.22 [95% CI = 9.72–16.73] vs. 21.53 [95% CI = 18.09–24.97], p = 0.001, 27.45 [95% CI = 22.23–32.69] vs. 20.78 [95% CI = 16.79–24.77], p = 0.040, and 31.78 [95% CI = 26.04–37.52] vs. 21.98 [95% CI = 18.29–25.67], p = 0.004, respectively) (Fig. 2e–f).
Humoral immune responses to inactivated SARS-CoV-2 vaccines in OPTB subgroups
The seropositivity rates of Abs against RBD-IgG and SARS-CoV-2 neutralizing Abs were similar in the OPTB patient groups with fibrosis and calcification (66.7% vs. 72.2%, p = 0.970, and 62.7% vs. 63.0%, p = 0.982, respectively), as were the Ab concentrations against RBD-IgG (median [IQR]: 2.41 (0.80–5.52) vs. 1.96 (0.88–4.59), respectively, p = 0.513) and SARS-CoV-2 neutralizing Abs (0.21 [0.11–0.31] vs. 0.19 [0.13–0.30], respectively, p = 0.788) (Fig. 3a–d).
a–d Antibody responses to inactivated SARS-CoV-2 vaccines in OPTB subgroups. The seropositivity rates (b, d) and concentrations of Abs (a, c) against RBD-IgG and SARS-CoV-2 neutralizing Abs in fibrosis (CT) group (n = 51) and calcification (CT) group (n = 54), respectively. Chi-square test and Mann–Whitney U test (interquartile range, IQR) were used for comparisons OPTB subgroups. The limit of detection range of concentrations of anti-RBD-IgG{0.375–1000AU/mL}, the limit of detection range of concentrations of CoV-2 NAb{0.05–30ug/mL}. The p-values represented in this figure are all adjusted p-values. CT, computed tomography
Furthermore, the frequencies of B cells, RBD-specific B cells, RBD+ resting MBCs, RBD+ activated MBCs, RBD+ atypical MBCs, RBD+ intermediate MBCs, RBD-specific MBCs, RBD-specific MBCs IgG+, and RBD-specific MBCs IgM+ were similar between the fibrosis and calcification subgroups (median [IQR], 6.16 [3.61–9.92] vs. 20.60 [14.45–23.20], p = 0.631, 19.35 [95% CI = 17.64–21.07] vs. 19.79 [95% CI = 18.08–21.50], p = 0.717, 12.30 [3.21–18.45] vs. 20.10 [13.65–30.05], p = 0.458, 19.30 [14.90–31.05] vs. 28.80 [21.60–45.40], p = 876, 33.30 [21.50–50.75] vs. 29.60 [5.00–43.85], p = 0.539, 28.70 [4.15–36.95] vs. 35.00 [29.80–48.15], p = 0.493, 35.00 [26.10–50.40] vs. 16.60 [12.60–19.90], p = 0.624, 17.20 [14.00–20.15] vs. 22.70 [14.40–35.60], p = 0.458, and 23.60 [95% CI = 19.89–27.31] vs. 23.67 [95% CI = 19.84–27.50], p = 0.980, respectively) (Supplementary Fig. 3a).
Humoral immune response to inactivated SARS-CoV-2 vaccines in OPTB subgroups < 55 and ≥ 55 years
There were no differences in serum concentrations of the two Abs between the fibrosis and calcification subgroups of OPTB patients aged < 55 and ≥ 55 years (6.66 [0.89–13.07] vs. 2.28 [0.56–4.66] vs. 1.22 [0.65–2.30], p = 0.059, and 0.38 [0.12–0.49] vs. 0.20 [0.11–0.30] vs. 0.18 [0.12–0.23], p = 0.262, respectively). Based on age (≥ 55 and < 55 years), there was no significant difference in the seropositivity rates for the two Abs between the calcification and fibrosis subgroups (75% vs. 69.2% vs. 55.6% vs. 80.6%, p = 0.284, and 66.7% vs. 61.5% vs. 55.6% vs. 66.7%, p = 0.864, respectively) (Supplementary Fig. 4a–d).
Based on age (≥ 55 and < 55 years), there were differences in the frequencies of B cells and RBD+ intermediate MBCs between the fibrosis and calcification subgroups (6.77 [2.94–9.99] vs. 5.45 [4.00–9.15] vs. 9.15 [7.36–12.75], p = 0.016, and 2.63 [0.82–32.00] vs. 31.10 [15.40–41.13] vs. 23.90 [1.23–36.63], p = 0.042, respectively) (Fig. 4a, b).
a–b RBD+-specific B cells responses to inactivated SARS-CoV-2 vaccines in OPTB subgroups < 55 and ≥ 55 years. Specific memory B cells (MBCs) responses to inactivated SARS-CoV-2 vaccines in fibrosis (CT) < 55 years (n = 12), fibrosis (CT) ≥ 55 years (n = 39), calcification (CT) < 55 years (n = 18), and calcification (CT) ≥ 55 years (n = 36). The frequencies of a B cells and b RBD+ intermediate MBCs. Kruskal–Wallis test was used for comparisons OPTB subgroups, and the results were corrected by Bonferroni. The p-values represented in this figure are all adjusted p-values. CT, computed tomograghy; MBCs, memory B cells
T cell responses to inactivated SARS-CoV-2 vaccines
The frequencies of CTLA-4+ cells were higher in OPTB patients than healthy controls (p = 0.023), while there were no difference in the frequencies of T lymphocytes, CD3+T lymphocytes, CD4+ T lymphocytes, and Tregs, in addition to cells positive for OX40, LAG-3, and PD-1 (Fig. 5a).
a T cell responses to inactivated SARS-CoV-2 vaccines. The frequencies (a) of Lc (T lymphocyte), CD3+T, CD4+T, Treg, OX40, CTLA-4+, LAG-3, and PD-1 in OPTB patients and healthy controls. Student’s t-test (confidence interval, CI)/Mann–Whitney U test (interquartile range, IQR) were used for two-group comparison (healthy controls and OPTB patients). The p-values represented in this figure are all adjusted p-values
As shown in Table 3, the time interval after full-course vaccination was the essential factor related to the poor response of Abs against RBD-IgG, while the counts of lymphocyte were a protective factor for Abs against RBD-IgG.
Discussion
More than 10 million new cases of PTB are reported each year, mostly in developing nations, such as China, which continues to rank third (8.4%) among the 30 nations with the highest incidence rate of PTB as of 2019 with 58 cases for every 10,000 persons (World Health Organization, 2019) [15]. Although considerable progress in lowering the incidence of PTB has been achieved in recent years, the disease has not yet been eliminated in China.
A prospective observational trial that examined the immunological response in this population found that inactivated SARS-CoV-2 vaccines were safe and effective against OPTB. The overall incidence of AEs within 7 days of vaccination was similar between OPTB patients and healthy controls at 10.7% for OPTB patients, which was comparable to healthy controls (11.3%), but lower than the phase I trial of the BNT162b2 mRNA COVID-19 vaccine (26.67%) and a phase II trial conducted by the National Institute of Allergy and Infectious Diseases of mRNA-1273 (27.33%) [16].
The results of the present study found that serum levels of Abs against RBG-IgG and SARS-CoV-2 neutralizing Abs following a full course of vaccination were significantly reduced in OPTB patients, consistent with the findings of a prior study [17]. In addition, seroconversion rates were reduced in OPTB patients. Approximately 26.88% of OPTB patients, as opposed to 13.21% of the healthy controls, failed to produce a sufficient immune response following immunization. These findings revealed that inactivated SARS-CoV-2 vaccinations had a limited immunogenic effect in OPTB patients.
MBCs are terminally developed immune cells that develop after exposure to an antigen [18, 19]. MBCs differentiate into Ab-secreting cells in response to subsequent infections [20, 21]. The frequencies of RBD+ atypical MBCs were increased during chronic inflammation and numerous investigations have shown a negative correlation between these proportions and the quantity of blood Abs [22, 23]. The frequencies of RBD-specific MBCs are reduced in response to various immunodeficiency disorders, suggesting positive correlations with Ab levels [24]. In contrast to the healthy controls, the frequencies of RBD+ atypical MBCs were increased in OPTB patients, while the frequencies of RBD-specific MBCs were decreased, suggesting that immune reactivation may be impaired in OPTB patients.
Inhibition of the immunoregulatory function of T cells is the principal negative regulator of T lymphocytes and CTLA-4+ cells, which are mostly generated by CD4+ CD25hi Tregs [25, 26]. Previous studies have reported that individuals with PTB produce significant amounts of CD4+ CD25hi Tregs at 8 weeks and 6 months after treatment [27, 28]. In addition, a prior study [29] revealed that the frequencies of total MBCs and transformed MBCs were significantly lower in the CTLA-4-Ig subgroup of rheumatoid arthritis patients than the control group and the peripheral blood concentrations of anti-RBD-IgG Abs were also significantly lower at 6 months after inoculation with SARS-CoV-2 vaccines, demonstrating that CTLA-4 indirectly lowers the generation of MBCs by directly suppressing T cell activity, which in turn impacts Ab concentrations. In the present study, OPTB patients had higher peripheral blood levels of CTLA-4 than the control group, which may be related to immunosuppression by Tregs.
The results of a previous clinical trial showed that immunogenicity was comparatively lower in older as compared to younger individuals after receiving the COVID-19 BNT162b2 vaccination, as evidenced by the Ab response [30]. In another study [31], the overall immune response was limited in individuals aged > 55 years following two doses of the inactivated CoronaVac vaccine. Intriguingly, a clinical trial of the mRNA-1273 COVID-19 vaccine found comparable immunological responses in older and younger individuals [32], while the ChAdOx1nCoV-19 vaccine induced comparable immune responses in younger and older patients [33, 34]. However, empirical data are required to evaluate the potential link between age and Ab responses to inactivated COVID-19 vaccines, as age may be not related to Ab responses.
The effect of the proportion of lymphocytes on the responses of Abs against RBD-IgG remains unclear, as two past studies found positive correlations between the responses of Abs against RBD-IgG and lymphocyte counts [35, 36], while another found no relationship [37]. In the present study, levels of Abs against RBD-IgG were linked with the lymphocyte counts. In addition, the length of time following complete vaccination was associated with the magnitude of the anti-RBD-IgG Ab response, which is in agreement with previous research [24, 38].
The strengths of this study are as follows: Notably, this was the first study to assess the safety and immunogenicity of SARS-CoV-2 vaccines in OPTB patients to provide evidence for clinical practice. Second, responses of two Abs were comprehensively analyzed to assess humoral and cellular immunity to vaccine. Third, the results confirmed that poor immune responses to Abs against RBD-IgG were related to the time interval after complete vaccination. However, there were several limitations to this study. First, this study only included data of up to 105 days post-vaccination. Second, T cells responses were measured in only a subset of participants. Thus, follow-up studies are needed. Third, travel restrictions due to intermittent local outbreaks of COVID-19 at 6 months after full immunization prohibited longitudinal investigations. Fourth, the sample size is rather small. Fifth, the results might not be generalizable to other vaccines, settings, or ethnicities.
In conclusion, this study comprehensively analyzed the safety and immunogenicity of COVID-19 inactivated vaccines for OPTB. The inactivated SARS-CoV-2 vaccine was well tolerated by OPTB patients and no severe AEs were reported. However, the humoral immune responses were weaker in OPTB patients.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Marasco V, Carniti C, Guidetti A, Farina L, Magni M, Miceli R et al (2022) T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol 196:548–558
Sy KTL, Haw NJL, Uy J (2020) Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond) 52:902–907
Liu Y, L Bi, Y Chen, Y Wang, M Cheng (2020) Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv
WHO (2021) Global tuberculosis report. Geneva, Switzerland: World Health Organization
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B et al (2021) Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 384:2187–201
John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D et al (2021) Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med 181:1306–1314
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J et al (2021) Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 385:320–329
Abreu MT, Carvalheiro H, Rodrigues-Sousa T, Domingos A, Segorbe-Luis A, Rodrigues-Santos P et al (2014) Alterations in the peripheral blood B cell subpopulations of multidrug-resistant tuberculosis patients. Clin Exp Med 14:423–429
Lyashchenko KP, Vordermeier HM, Waters WR (2020) Memory B cells and tuberculosis. Vet Immunol Immunopathol 221:110016
Mak WA, Koeleman JGM, van der Vliet M, Keuren F, Ong DSY (2022) SARS-CoV-2 antibody and T cell responses one year after COVID-19 and the booster effect of vaccination: a prospective cohort study. J Infect 84:171–178
Serrano CJ, Castañeda-Delgado JE, Trujillo-Ochoa JL, González-Amaro R, García-Hernández MH, Enciso-Moreno JA (2015) Regulatory T-cell subsets in response to specific Mycobacterium tuberculosis antigens in vitro distinguish among individuals with different QTF and TST reactivity. Clin Immunol 157:145–155
Dyatlov AV, Apt AS, Linge IA (2019) B lymphocytes in anti-mycobacterial immune responses: pathogenesis or protection? Tuberculosis (Edinb) 114:1–8
Zhang J, Zhang Y, Yang Q, Deng G, Chen Qi, Chen W et al (2019) Dynamic changes of T cell and B cell subsets in peripheral blood of tuberculosis patients during treatment. China Trop Med 19:7
Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V et al (2016) Patients with tuberculosis have a dysfunctional circulating B-cell compartment, which normalizes following successful treatment. PLoS Pathog 12:e1005687
WHO (2019) Global tuberculosis report. Geneva, Switzerland: World Health Organization
Feng J, Zhang J (2021) Research progress in COVID-19 vaccines. Anti Infect Pharm. 1715–1723
Harboe ZB, Hamm SR, Pérez-Alós L, Sivapalan P, Priemé H, Wilcke T, et al. (2022) Antibody responses and risk factors associated with impaired immunological outcomes following two doses of BNT162b2 COVID-19 vaccination in patients with chronic pulmonary diseases, BMJ Open Respir Res, 9
Lau D, Lan LY, Andrews SF, Henry C, Rojas KT, Neu KE et al (2017) Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol 2:eaai8153
Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, Romero-Ramírez S, Páez-Franco JC, Meza-Sánchez DE, et al. (2020) B cell subsets as severity-associated signatures in COVID-19 patients. Front Immunol; 11
Terreri S, Piano Mortari E, Vinci MR, Russo C, Alteri C, Albano C et al (2022) Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 30:400-408.e4
Wang CY, Hwang KP, Kuo HK, Peng WJ, Shen YH, Kuo BS, et al. (2022) A multitope SARS–CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J Clin Invest; 132
Oliviero B, Varchetta S, Mele D, Mantovani S, Cerino A, Perotti CG et al (2020) Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol Immunol 17:1101–1103
Wildner NH, Ahmadi P, Schulte S, Brauneck F, Kohsar M, Lütgehetmann M et al (2021) B cell analysis in SARS-CoV-2 versus malaria: increased frequencies of plasmablasts and atypical memory B cells in COVID-19. J Leukoc Biol 109:77–90
Ao L, Lu T, Cao Y, Chen Z, Wang Y, Li Z et al (2022) Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect 11:1126–1134
Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A et al (2020) Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med 383:2439–2450
Medeiros GX, Sasahara GL, Magawa JY, Nunes JPS, Bruno FR, Kuramoto AC et al (2022) Reduced T cell and antibody responses to inactivated coronavirus vaccine among individuals above 55 years old. Front Immunol 13:812126
Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M et al (2020) Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383:2427–2438
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR et al (2021) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396:1979–1993
Angel-Korman A, Peres E, Bryk G, Lustig Y, Indenbaum V, Amit S et al (2022) Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel: the case for a third vaccine dose. Clin Kidney J 15:226–234
Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R et al (2021) T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med 27:270–278
Wang P, Yang N, Xue Y, Zhou J, Wu Y, Wang T, et al. (2022) The fluctuation trend of serum anti-SARS-CoV-2 IgM/IgG antibodies seroprevalence in the non-COVID-19 infected population and correlation with peripheral blood leukocyte parameters in Beijing, China, 2021: a real-world study. Vaccines (Basel). 10
Margalit O, Shacham-Shmueli E, Itay A, Berger R, Halperin S, Jurkowicz M et al (2022) Seropositivity and neutralising antibodies at six months after BNT162b2 vaccination in patients with solid tumours. Eur J Cancer 168:51–55
Barin B, Kasap U, Selçuk F, Volkan E, Uluçkan Ö (2022) Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study. Lancet Microbe 3:e274–e283
Thye T, Scarisbrick G, Browne EN, Chinbuah MA, Gyapong J, Osei I et al (2009) CTLA4 autoimmunity-associated genotype contributes to severe pulmonary tuberculosis in an African population. PLoS ONE 4:e6307
Sansom DM, Walker LS (2006) The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev 212:131–148
Feruglio SL, Kvale D, Dyrhol-Riise AM (2017) T cell responses and regulation and the impact of in vitro IL-10 and TGF-β modulation during treatment of active tuberculosis. Scand J Immunol 85:138–146
Roberts T, Beyers N, Aguirre A, Walzl G (2007) Immunosuppression during active tuberculosis is characterized by decreased interferon- gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor- beta, and interleukin-4 mRNA levels. J Infect Dis 195:870–878
Farroni C, Picchianti-Diamanti A, Aiello A, Nicastri E, Laganà B, Agrati C et al (2022) Kinetics of the B- and T-cell immune responses after 6 months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front Immunol 13:846753
Acknowledgements
We thank the health care center and department of clinical laboratory of the Second Affiliated Hospital, Chongqing Medical University for their support.
Funding
This work is supported by the National Science and Technology Major Project of China (2017ZX10202203-007, 2017ZX10202203-008, 2018ZX10302206-003) and a pilot project of clinical cooperation between traditional Chinese and western medicine for significant and complicated diseases of National Administration of Traditional Chinese Medicine: hepatic fibrosis. We also acknowledge the support of the National Natural Science Foundation of China (81772198), the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389), and the kuanren talents program of the second affiliated hospital of Chongqing medical University.
Author information
Authors and Affiliations
Contributions
Concept and design: Hong Ren, DePeng Jiang. Funding acquisition: Hong Ren. Participant recruitment and characterization: Lei Yang, Feng Xiang, DePeng Jiang, Hong Ren. Experiment execution: Feng Xiang. Acquisition, analysis or interpretation of data: Lei Yang, DePeng Jiang, Hong Ren. Supervision: DePeng Jiang, Hong Ren. Administrative support: Hong Ren. Drafting and critical revision of manuscript: Lei Yang, Dian Wang, Qiao Guo, Bing Deng, DePeng Jiang, Hong Ren. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval statement for human and/or animal studies
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University and conformed with the ethical guidelines of the Declaration of Helsinki.
Patient consent statement
Written informed consent was obtained from all participants prior to their inclusion in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Yang and Feng Xiang are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L., Xiang, F., Wang, D. et al. The safety and immunogenicity of inactivated COVID-19 vaccine in old pulmonary tuberculosis patients. Eur J Clin Microbiol Infect Dis 42, 503–512 (2023). https://doi.org/10.1007/s10096-023-04566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04566-0