Abstract
Leishmaniasis is a parasitic infection expressing different clinical phenotypes. Visceral leishmaniasis (VL) is considered an opportunistic infection among people with human immunodeficiency virus (HIV). The objective of this review was to identify published data on the prevalence of Leishmania spp. infection among PWH and to define particular determinants that affect critically the epidemiological characteristics of VL-HIV coinfection and, potentially, its burden on public health. Two independent reviewers conducted a systematic literature search until June 30, 2022. Meta-analyses were conducted using random-effects models to calculate the summary prevalence and respective 95% confidence intervals (CI) of leishmaniasis among PWH. Meta-regression analysis was performed to investigate the impact of putative effect modifiers, such as the mean CD4 cell count, on the major findings. Thirty-four studies were eligible, yielding a summary prevalence of 6% (95%CI, 4–11%) for leishmaniasis (n = 1583) among PWH (n = 85,076). Higher prevalence rates were noted in Asia (17%, 95%CI, 9–30%) and America (9%, 95%CI, 5–17%) than in Europe (4%, 95%CI, 2–8%). Prevalence rates were significantly mediated by the age, sex, and CD4 cell count of participants. Heterogeneity remained significant in all meta-analyses (p < 0.0001). In the majority of included studies, people were coinfected with HIV and Leishmania species associated with VL, as opposed to those associated with cutaneous leishmaniasis. No sign of publication bias was shown (p = 0.06). Our summary of published studies on leishmaniasis among PWH is important to provide prevalence estimates and define potential underlying factors that could guide researchers to generate and further explore specific etiologic hypotheses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a vector-borne parasitic infection with three primary clinical forms: visceral leishmaniasis (VL), cutaneous leishmaniasis, and mucocutaneous leishmaniasis [1]. More than 20 distinct species of the genus Leishmania are responsible for the clinical manifestations of the disease. The global incidence of leishmaniasis is estimated at 700,000 to 1 million new cases per year [2, 3]. Though the clinical manifestations of Leishmania infection are traditionally classified into three syndromes, immunocompromised people are more likely to have disseminated disease [1]. Leishmania species that typically cause only cutaneous disease could cause disseminated disease in people with compromised cellular immunity such as people with human immunodeficiency virus (HIV (PWH)).
Over the last decade, advances in healthcare delivery have led to substantial decreasing trends in VL although it remains a fatal disease in endemic areas, such as in some African regions [2, 4]. VL is considered an opportunistic infection for PWH in countries/regions, where leishmaniasis is endemic because coinfection rates are much higher in Leishmania-endemic countries/regions [5]. As of 2021, Leishmania-HIV coinfections have been reported from 45 countries [6, 7]. High Leishmania-HIV coinfection rates have been noted in Brazil, Ethiopia, and the state of Bihar in India [8,9,10,11]. Leishmania-HIV coinfected people are more likely to develop the disseminated clinical disease, as well as high relapse and mortality rates [6]. Due to the high prevalence of Leishmania-HIV coinfection in these countries and the lack of a single reliable serologic test for leishmaniasis diagnosis, screening for Leishmania coinfection with a combination of laboratory tests would be useful for PWH living in or originating from endemic regions [12]. From a therapeutic perspective, the critical role of highly active antiretroviral therapy (HAART) in the outcome of VL-HIV coinfection has been clearly reported [13]. In particular, once Leishmania-HIV coinfection is diagnosed, early initiation of HAART is recommended as a protective modality against VL relapses. Antiretroviral treatment reduces the development of the disease, delays relapses, and increases the survival of the coinfected patients [14]. Several reviews have synthesized the existing evidence around the prevalence of Leishmania-HIV coinfection, as well as the demographic and diagnostic characteristics of the disease [15,16,17]. For instance, Lindoso et al. reported that VL occurs in 12 countries of Latin America, with 96% of cases reported in Brazil [15]. Moreover, Boelaert et al. showed that specific immunochromatographic test, i.e., rK39 ICT, shows high sensitivity and specificity for the diagnosis of visceral leishmaniasis in patients with febrile splenomegaly and no previous history of the disease, but the sensitivity is notably lower in east Africa than in the Indian subcontinent [16].
Overall, the results of the present study is expected to be useful to public health authorities when implementing better control strategies and guiding future developments in the field. We reviewed published evidence on the prevalence rates of Leishmania spp. among PWH and to quantitatively synthesize the results of published studies.
Methods
Search strategy and study selection
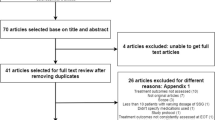
A literature search of Medline database was conducted from inception up to June 30th, 2022, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table 1) [18]. Two reviewers performed the literature search independently using the algorithm (leishmania OR leishmaniasis) AND (immunocompromised OR immunodeficiency OR HIV OR AIDS OR (immune AND deficiency)) AND (incidence OR prevalence OR rate). Both reviewers evaluated the reference lists of all identified eligible studies using the “snowball” procedure, which entails searching the reference lists of each manuscript for additional eligible articles potentially missed from the initial literature search. We included articles that examined specifically the prevalence rates of leishmaniasis among PWH. No language or other restrictions were applied. Case reports and experimental or animal studies were excluded. Following the literature search, duplicate citations were removed, and the remaining articles were independently screened by two investigators to identify studies that met the pre-determined inclusion criteria. The study selection was conducted in two stages. First, we reviewed article titles and abstracts and removed those that did not meet our inclusion/exclusion criteria. Second, we retrieved and reviewed the full text of the remaining articles. For the remaining studies, the full papers were retrieved for further screening. In case of disagreement in the selection of studies or snowball procedure, the final decision was reached by team consensus. In articles with overlapping populations, the most recent or most complete publication was considered eligible.
Data extraction
For each eligible publication, the following study variables were extracted: publication year, country/region where the study was performed, study design, study period, follow-up period, sample size, age at diagnosis, mean patient’s CD4 count, and the proportion of males. In addition, information about the number of patients with Leishmania-HIV coinfection, the type of Leishmania infection, type of Leishmania diagnostic method, the treatment administered, and the outcome of such infection were also extracted. Two reviewers performed the data extraction, and any disagreements were resolved by consensus.
Statistical analysis
A descriptive presentation of the eligible studies was initially performed (Table 1). Thereafter, the prevalence of Leishmania infection among PWH and the respective 95% confidence intervals (CI) were extracted or calculated from the available data using the Wilson’s method [19]. Meta-analyses were undertaken using random-effects models [20] to estimate the prevalence of Leishmania-HIV coinfection overall and by study region (Europe, America, and Asia). We performed sub-analyses of prevalence by region where numbers allowed. Between-study heterogeneity was assessed using the Cochran Q and I2 statistics20. The Z-test was applied for the overall effect, and statistical significance was set at p < 0.10. In addition, we performed sensitivity meta-analyses excluding a study per time to identify whether heterogeneity was affected by the inclusion of a specific study. The impact of potential effect modifiers, such as publication year, mean age, percent of males, and mean CD4 cell count on the main results, was explored in meta-regression analysis. Publication bias was calculated using the Egger’s test (significance level was set at p < 0.05) [21]. Analyses were performed using the Stata software.
Results
Characteristics of the studies
Figure 1 shows the results of the literature search and selection process. Following the exclusion of publications with overlapping populations, a total of 34 eligible studies were finally included in this analysis [12, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
The descriptive characteristics of the included studies are presented in Table 1. Nineteen of the studies were of cohort design, and the remaining fifteen were found to be of cross-sectional design. The mean follow-up period ranged between 0.5 and 3.8 years. Most studies were conducted in Europe (France, Spain, Italy; n = 18), followed by America (Brazil, USA; n = 9), Asia (Iran, Thailand; n = 6), and Africa (Morocco; n = 1).
Prevalence of leishmaniasis among PWH
Thirty-four eligible studies yielded a total sample of 85,076 PWH, of which leishmaniasis was diagnosed in 1583 people. The average percent of males was 72.4%, while the mean age of participants ranged from 30 to 41 years (median 36.9 years). Diagnosis of leishmaniasis was established through bone marrow biopsy and/or blood tests in the majority of studies (Table 1). The prevalence of leishmaniasis was found to be particularly variable across regions globally, although a summary prevalence of leishmaniasis among PWH was estimated as high as 6% (95% CI, 4–11%; n = 34 studies) with significant between-study heterogeneity (I2, 99.3%, p < 0.0001) (Fig. 2). The prevalence was 9% among studies conducted in America (95% CI, 5–17%; n = 9 studies) (Fig. 3a) and even higher among those conducted in Asia (17%, 95% CI, 9–30%; n = 6 studies) (Fig. 3b) compared to the prevalence calculated for European studies (4%, 95% CI, 2–8%; n = 18 studies) (Fig. 3c). Heterogeneity remained significant in the subgroup meta-analyses by study origin.
Forest plot showing the prevalence of Leishmania-HIV coinfection. Prevalence ratios of individual studies are indicated by the data markers; shaded boxes around data markers reflect the statistical weight of the study; 95% confidence intervals (CI) are indicated by the error bars; summary-effect estimates with their 95% CI are depicted as a diamond
Forest plot showing the prevalence of Leishmania-HIV coinfection by study origin: a America, b Asia, and c Europe. Prevalence ratios of individual studies are indicated by the data markers; shaded boxes around data markers reflect the statistical weight of the study; 95% confidence intervals (CI) are indicated by the error bars; summary-effect estimates with their 95% CI are depicted as a diamond
In the majority of included studies, people were diagnosed with HIV and Leishmania species associated with VL, as opposed to Leishmania species associated with cutaneous leishmaniasis, with the exception of one study where 25 out of 27 Leishmania-HIV infected participants had VL, and the remaining two had cutaneous leishmaniasis [41].
In the meta-regression analyses, age (p = 0.05) and CD4 cell count (p = 0.02) were statistically significant positive modifiers of the observed associations, whereas sex (percent of males) had a significant inverse effect modification (p = 0.002). Publication year had a borderline mediating effect on the results of the main meta-analyses (p = 0.06) (Table 2). Sensitivity meta-analyses excluding a study per time showed similar results with those of the main analyses, without reducing the between-study heterogeneity. Egger’s test showed no sign of publication bias (p = 0.06) (Supplementary Fig. 1). Information regarding treatment of Leishmania-HIV individuals was reported in 11 studies only. Liposomal amphotericin B, conventional pentavalent antimonial, pentamidine, allopurinol, itraconazole, and aminosidine were reported as medications; amphotericin B was the most frequently used medication.
Discussion
The present systematic review and meta-analysis of 34 published studies including 1583 individuals with Leishmania-HIV coinfection provides evidence for 6% prevalence of leishmaniasis among PWH living in or from populations of Leishmania-endemic regions. It should not be overlooked, however, that the abovementioned prevalence practically reflects the overall frequency in VL-PWH in the specific countries identified and included in the present study. As it was expected, we observed higher prevalence in studies from Asia and the Americas compared to studies from Europe; data on leishmaniasis among PWH were unavailable for other regions where Leishmania is endemic including Africa and the Middle East. In addition, the present results are consistent with the already well-described caveat that the prevalence of species causing VL is higher in regions (i.e., the “Old World”) where such species are endemic and regions with migration from areas where such species are endemic. Similarly, we found the prevalence of cutaneous and mucocutaneous leishmaniasis to be the highest in regions where the species causing these disease manifestations are endemic (e.g., the “New World”).
Prevalence rates varied significantly by age, sex, and CD4 cell count of participants. Publication year also had a borderline significant mediating effect, which could be potentially explained by treatment advances, i.e., the increased availability of ART as time progressed. Overall, the results were based on highly heterogeneous studies mainly conducted in Europe. Indeed, the variation of findings by the study setting in variable time periods or different counties points to a very complex interplay between a wide range of environmental and genetic factors.
Leishmaniasis is considered an opportunistic infection among PWH, especially among those living in VL-endemic sub-tropical and tropical regions around the world, including the Mediterranean [55]. Globally, the occurrence of Leishmania-HIV coinfection has been enhanced by the spread of HIV into rural areas and the concurrent spread of leishmaniasis to suburban/urban areas [56, 57]. Some experts expect the number of cases of Leishmania-HIV coinfection will increase as the distributions of the two infections further overlap [58]. In several Southwestern European countries, such as France, Italy, Spain, and Portugal, increasing incidence rates of Leishmania-HIV coinfection have been noted, with most cases concerning VL [57].
Studies on the immunological background of Leishmania-HIV coinfection report that both microorganisms infect and multiply within myeloid or lymphoid cells, enhancing thus the reciprocal modulation of Leishmania and HIV pathogenesis [57]. Additionally, given that recovery from leishmaniasis is associated with long-term persistence of parasites at the primary sites of infection and their draining lymph nodes, it is likely that HIV-mediated immunosuppression due to CD4( +) T cell depletion could lead to reactivation leishmaniasis, particularly in immunocompromised patients [59,60,61]. Indeed, the present results suggest a potential prevalence effect modification due to the PWH population control of HIV infection, which CD4 count is a surrogate marker for. Of note, the mean CD4 count for nearly all cohorts was less than 200, a finding clearly carrying increased risk of leishmaniasis clinical manifestations.
The lethality of VL increases by 4.6% to 16.6% when associated with HIV infection [62]. The presence of HIV can lead to severe forms of VL, which are difficult to control and manage. Furthermore, PWH are more likely to have severe and/or prolonged VL with a more difficulty treatment course, which further may increase the HIV replication and the clinical evolution of AIDS [62]. Therefore, this coinfection warrants concern and must be recognized and treated in a timely manner. Recent reports from the World Health Organization recommend that patients with Leishmania-HIV coinfection should be treated with liposomal amphotericin B, whereas early administration of HAART acts as a protective modality against VL relapse [6, 55].
The sound methodological approach has been a strength of the current study given that meta-analyses, sub-analyses by study origin, sensitivity meta-analyses, and meta-regression analyses were run. However, the present results should be cautiously interpreted in view of limitations inherent to the study design and data availability of eligible studies including variable criteria for selection of the comparison groups, statistical analysis methods, and variable follow-up periods.
The highly heterogeneous results across the included studies could either indicate that different factors may be implemented in the prevalence of Leishmania-HIV coinfection in each country or could point to the heterogeneity of the methodological approaches, e.g., in statistical analysis, implemented by each study. Moreover, information on other variables of interest, such as ART at time of leishmaniasis diagnosis, mortality of people with Leishmania-HIV coinfection, and use of secondary chemoprophylaxis post-leishmaniasis treatment, were available by only 1–2 studies, thus not allowing generalization of the results.
Although the articles included in the present study are biased towards specific regions (Europe and Brazil) and cases visceral leishmaniasis only, it becomes evident that the field of VL in HIV patients remains of particular scientific interest. Further studies, both retrospective and prospective, should be specially designed and conducted in order the role of critical individual determinants of the disease to be defined and the between-country comparisons to be feasible.
Conclusions
In view of the still rising rates of the HIV/AIDS pandemic in some parts of the world, we noted a high prevalence of Leishmania infection among PWH. Heterogeneity issues and diverse study settings should be taken into account when interpreting the results of the present study. However, and beyond the above factors, this attempt to summarize current findings is of significant importance in providing effect estimates, as well as to point towards potential underlying factors. Further research based on well-designed studies is needed to generate and explore specific etiological hypotheses, as well as to compare prevalence and determinants between countries reliably and effectively.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to potential compromise of individual privacy but are available from the corresponding author on reasonable request.
Code availability
Not applicable for this submission.
References
Handler MZ, Patel PA, Kapila R, Al-Qubati Y, Schwartz RA (2015) Cutaneous and mucocutaneous leishmaniasis: clinical perspectives. J Am Acad Dermatol 73(6):897–908. https://doi.org/10.1016/j.jaad.2014.08.051
Burza S, Croft SL, Boelaert M (2018) Leishmaniasis. Lancet 392(10151):951–970. https://doi.org/10.1016/S0140-6736(18)31204-2
Ruiz-Postigo JA, Jain S, Mikhailov A, Maia-Elkhoury AN, Valadas S, Warusavithana S, Osman M, Lin Z, Beshah A, Yajima A, Gasimovg E (2021) Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap. WHO, Weekly Epidemiological Register 35(96):401–420
Alvar J, Yactayo S, Bern C (2006) Leishmaniasis and poverty. Trends Parasitol 22(12):552–557. https://doi.org/10.1016/j.pt.2006.09.004
van Griensven J, Carrillo E, López-Vélez R, Lynen L, Moreno J (2014) Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 20(4):286–299. https://doi.org/10.1111/1469-0691.12556
WHO. WHO website—Leishmaniasis: fact sheets. 2019. Available: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis
Charoensakulchai S, Bualert L, Manomat J et al (2020) Risk factors of Leishmania infection among HIV-infected patients in Trang Province, Southern Thailand: a study on three prevalent species. Am J Trop Med Hyg 103(4):1502–1509. https://doi.org/10.4269/ajtmh.20-0332
Daher EF, Fonseca PP, Gerhard ES, Silva Leitão TMJ, Silva Júnior GB (2009) Clinical and epidemiological features of visceral leishmaniasis and HIV co-infection in fifteen patients from Brazil. J Parasitol 95(3):652–655. https://doi.org/10.1645/GE-1678.1
ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN (2008) Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis 46(11):1702–1709. https://doi.org/10.1086/587899
Redhu NS, Dey A, Balooni V, Singh S (2006) Leishmania–HIV co-infection: an emerging problem in India. AIDS 20(8):1213–1215. https://doi.org/10.1097/01.aids.0000226971.42622.4e
Cloots K, Marino P, Burza S, Gill N, Boelaert M, Hasker E (2021) Visceral leishmaniasis-HIV coinfection as a predictor of Increased Leishmania transmission at the village level in Bihar, India. Front cell infect Microbiol 11. https://doi.org/10.3389/fcimb.2021.604117
Guedes DL, Justo AM, Barbosa Júnior WL et al (2021) Asymptomatic Leishmania infection in HIV-positive outpatients on antiretroviral therapy in Pernambuco, Brazil. PLoS Negl Trop Dis 15(1):e0009067. https://doi.org/10.1371/journal.pntd.0009067
Abongomera C, Diro E, Vogt F et al (2017) The risk and predictors of visceral leishmaniasis relapse in human immunodeficiency virus-coinfected patients in Ethiopia: a retrospective cohort study. Clin Infect Dis 65(10):1703–1710. https://doi.org/10.1093/cid/cix607
Cota GF, de Sousa MR, Rabello A (2011) Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis 5(6):e1153. https://doi.org/10.1371/journal.pntd.0001153
Lindoso JA, Cota GF, da Cruz AM et al (2014) Visceral leishmaniasis and HIV coinfection in Latin America. PLoS Negl Trop Dis 8(9):e3136. https://doi.org/10.1371/journal.pntd.0003136
Boelaert M, Verdonck K, Menten J, et al (2014) Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database of Systematic Reviews. Published online June 20 https://doi.org/10.1002/14651858.CD009135.pub2
Ibarra-Meneses AV, Corbeil A, Wagner V, Onwuchekwa C, Fernandez-Prada C (2022) Identification of asymptomatic Leishmania infections: a scoping review. Parasit Vectors 15(1):5. https://doi.org/10.1186/s13071-021-05129-y
Moher D (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med 151(4):264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17(8):857–872. https://doi.org/10.1002/(SICI)1097-0258(19980430)17:8%3c857::AID-SIM777%3e3.0.CO;2-E
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139–145. https://doi.org/10.1016/j.cct.2015.09.002
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Cunha MA, Celeste BJ, Kesper N et al (2020) Frequency of Leishmania spp infection among HIV-infected patients living in an urban area in Brazil: a cross-sectional study. BMC Infect Dis 20(1):885. https://doi.org/10.1186/s12879-020-05622-2
Charoensakulchai S, Bualert L, Manomat J et al (2020) Risk factors of Leishmania infection among HIV-infected patients in Trang Province, Southern Thailand: a study on three prevalent species. Am J Trop Med Hyg 103(4):1502–1509. https://doi.org/10.4269/ajtmh.20-0332
Barbosa Júnior WL, Justo AM, dos Santos AMA et al (2020) SLC11A1 (rs3731865) polymorphism and susceptibility to visceral leishmaniasis in HIV-coinfected patients from Northeastern Brazil. Parasitol Res 119(2):491–499. https://doi.org/10.1007/s00436-019-06596-0
Echchakery M, Nieto J, Boussaa S et al (2018) Asymptomatic carriers of Leishmania infantum in patients infected with human immunodeficiency virus (HIV) in Morocco. Parasitol Res 117(4):1237–1244. https://doi.org/10.1007/s00436-018-5805-y
Rezaei Z, Sarkari B, Dehghani M, Layegh Gigloo A, Afrashteh M (2018) High frequency of subclinical Leishmania infection among HIV-infected patients living in the endemic areas of visceral leishmaniasis in Fars province, southern Iran. Parasitol Res 117(8):2591–2595. https://doi.org/10.1007/s00436-018-5949-9
Pandey N, Siripattanapipong S, Leelayoova S et al (2018) Detection of Leishmania DNA in saliva among patients with HIV/AIDS in Trang Province, southern Thailand. Acta Trop 185:294–300. https://doi.org/10.1016/j.actatropica.2018.06.006
Guedes DL, Medeiros Z, Dionísio da Silva E et al (2018) Visceral leishmaniasis in hospitalized HIV-infected patients in Pernambuco Brazil. Am J Trop Med Hyg 99(6):1541–1546. https://doi.org/10.4269/ajtmh.17-0787
Manomat J, Leelayoova S, Bualert L et al (2017) Prevalence and risk factors associated with Leishmania infection in Trang Province, southern Thailand. PLoS Negl Trop Dis 11(11):e0006095. https://doi.org/10.1371/journal.pntd.0006095
Shafiei R, Mohebali M, Akhoundi B et al (2014) Emergence of co-infection of visceral leishmaniasis in HIV-positive patients in northeast Iran: a preliminary study. Travel Med Infect Dis 12(2):173–178. https://doi.org/10.1016/j.tmaid.2013.09.001
Ena J, Pasquau F, del Mar L-P, Martinez-Peinado C, Arjona F (2014) Screening for subclinical Leishmania infection in HIV-infected patients living in eastern Spain. Pathog Glob Health 108(8):356–361. https://doi.org/10.1179/2047773214Y.0000000164
Salvador F, Molina I, Sulleiro E et al (2013) Tropical diseases screening in immigrant patients with human immunodeficiency virus infection in Spain. Am J Trop Med Hyg 88(6):1196–1202. https://doi.org/10.4269/ajtmh.12-0714
Orsini M, Canela JR, Disch J et al (2012) High frequency of asymptomatic Leishmania spp infection among HIV-infected patients living in endemic areas for visceral leishmaniasis in Brazil. Trans R Soc Trop Med Hyg 106(5):283–288. https://doi.org/10.1016/j.trstmh.2012.01.008
Nascimento ET, Moura MLN, Queiroz JW et al (2011) The emergence of concurrent HIV-1/AIDS and visceral leishmaniasis in Northeast Brazil. Trans R Soc Trop Med Hyg 105(5):298–300. https://doi.org/10.1016/j.trstmh.2011.01.006
Abellán-Martínez J, Guerra-Vales JM, Fernández-Cotarelo MJ, González-Alegre MT (2009) Evolution of the incidence and aetiology of fever of unknown origin (FUO), and survival in HIV-infected patients after HAART (Highly Active Antiretroviral Therapy). Eur J Intern Med 20(5):474–477. https://doi.org/10.1016/j.ejim.2009.01.004
Carranza-Tamayo CO, de Assis TSM, Neri ATB, Cupolillo E, Rabello A, Romero GAS (2009) Prevalence of Leishmania infection in adult HIV/AIDS patients treated in a tertiary-level care center in Brasilia, Federal District, Brazil. Trans R Soc Trop Med Hyg 103(7):743–748. https://doi.org/10.1016/j.trstmh.2009.01.014
Colomba C, Saporito L, Vitale F et al (2009) Cryptic Leishmania infantum infection in Italian HIV infected patients. BMC Infect Dis 9:199. https://doi.org/10.1186/1471-2334-9-199
Soares VYR, Lúcio Filho CEP, de Carvalho LIM, de Morais a Silva AMM, Eulálio KD (2008) Clinical and epidemiological analysis of patients with HIV/AIDS admitted to a reference hospital in the northeast region of Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo 50(6):327–332. https://doi.org/10.1590/s0036-46652008000600003
García-García JA, Martín-Sánchez J, Gállego M et al (2006) Use of noninvasive markers to detect Leishmania infection in asymptomatic human immunodeficiency virus-infected patients. J Clin Microbiol 44(12):4455–4458. https://doi.org/10.1128/JCM.00921-06
Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK (2004) Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis 4:52. https://doi.org/10.1186/1471-2334-4-52
Russo R, Nigro L, Panarello G, Montineri A (2003) Clinical survey of Leishmania/HIV co-infection in Catania, Italy: the impact of highly active antiretroviral therapy (HAART). Ann Trop Med Parasitol 97(1):149–155. https://doi.org/10.1179/000349803225002624
del Giudice P, Mary-Krause M, Pradier C et al (2002) Impact of highly active antiretroviral therapy on the incidence of visceral leishmaniasis in a French cohort of patients infected with human immunodeficiency virus. J Infect Dis 186(9):1366–1370. https://doi.org/10.1086/344325
de La Rosa R, Pineda JA, Delgado J et al (2002) Incidence of and risk factors for symptomatic visceral leishmaniasis among human immunodeficiency virus type 1-infected patients from Spain in the era of highly active antiretroviral therapy. J Clin Microbiol 40(3):762–767. https://doi.org/10.1128/JCM.40.3.762-767.2002
Pintado V, Martín-Rabadán P, Rivera ML, Moreno S, Bouza E (2001) Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients. Comp Stud Med 80(1):54–73. https://doi.org/10.1097/00005792-200101000-00006
Bernabeu-Wittel M, Villanueva JL, Pachón J et al (1999) Etiology, clinical features and outcome of splenic microabscesses in HIV-infected patients with prolonged fever. Eur J Clin Microbiol Infect Dis 18(5):324–329. https://doi.org/10.1007/pl00015013
Dereure J, Pratlong F, Reynes J, Basset D, Bastien P, Dedet JP (1998) Haemoculture as a tool for diagnosing visceral leishmaniasis in HIV-negative and HIV-positive patients: interest for parasite identification. Bull World Health Organ 76(2):203–206
Pineda JA, Gallardo JA, Macías J et al (1998) Prevalence of and factors associated with visceral leishmaniasis in human immunodeficiency virus type 1-infected patients in southern Spain. J Clin Microbiol 36(9):2419–2422. https://doi.org/10.1128/JCM.36.9.2419-2422.1998
Benito N (1997) Bone marrow biopsy in the diagnosis of fever of unknown origin in patients with acquired immunodeficiency syndrome. Arch Intern Med 157(14):1577. https://doi.org/10.1001/archinte.1997.00440350085008
Lozano F, Torre-Cisneros J, Bascuñana A et al (1996) Prospective evaluation of fever of unknown origin in patients infected with the human immunodeficiency virus Grupo Andaluz para el Estudio de las Enfermedades Infecciosas. Eur J Clin Microbiol Infect Dis 15(9):705–711. https://doi.org/10.1007/BF01691956
Gradoni L, Scalone A, Gramiccia M, Troiani M (1996) Epidemiological surveillance of leishmaniasis in HIV-1-infected individuals in Italy. AIDS 10:785–792. https://doi.org/10.1097/00002030-199606001-00014
Dereure J, Reynes J, Pratlong F (1995) Visceral leishmaniasis in HIV-infected patients in the south of France. Bull World Health Organ 73(2):245–246
Miralles P, Moreno S, Perez-Tascon M, Cosin J, Diaz MD, Bouza E (1995) Fever of uncertain origin in patients infected with the human immunodeficiency virus. Clin Infect Dis 20(4):872–875. https://doi.org/10.1093/clinids/20.4.872
Bissuel F, Leport C, Perronne C, Longuet P, Vilde JL (1994) Fever of unknown origin in HIV-infected patients: a critical analysis of a retrospective series of 57 cases. J Intern Med 236(5):529–535. https://doi.org/10.1111/j.1365-2796.1994.tb00840.x
Amela C, López-Gay D, Alberdi JC, Castilla J (1996) Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur J Epidemiol 12(1):91–92. https://doi.org/10.1007/BF00144435
Alvar J, Aparicio P, Aseffa A et al (2008) The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21(2):334–359. https://doi.org/10.1128/CMR.00061-07
Curtin JM, Aronson NE (2021) Leishmaniasis in the United States: emerging issues in a region of low endemicity. Microorganisms 9(3):578. https://doi.org/10.3390/microorganisms9030578
Okwor I, Uzonna JE (2013) The immunology of Leishmania/HIV co-infection. Immunol Res 56(1):163–171. https://doi.org/10.1007/s12026-013-8389-8
Salih OMM, Nail A, Modawe G et al (2020) Risk factors of inpatients mortality of visceral leishmaniasis, Khartoum State, Sudan. J Global Infect Dis 12(3):135. https://doi.org/10.4103/jgid.jgid_25_20
Olivier M, Badaró R, Medrano FJ, Moreno J (2003) The pathogenesis of Leishmania/HIV co-infection: cellular and immunological mechanisms. Ann Trop Med Parasitol 97(sup1):79–98. https://doi.org/10.1179/000349803225002561
Santos-Oliveira JR, Giacoia-Gripp CB, Alexandrino de Oliveira P et al (2010) High levels of T lymphocyte activation in Leishmania-HIV-1 co-infected individuals despite low HIV viral load. BMC Infect Dis 10(1):358. https://doi.org/10.1186/1471-2334-10-358
Kubar J, Marty P, Lelièvre A et al (1998) Visceral leishmaniosis in HIV-positive patients. AIDS 12(16):2147–2153. https://doi.org/10.1097/00002030-199816000-00009
Machado CAL, Sevá da AP, Silva AAFA e, Horta MC (2021) Epidemiological profile and lethality of visceral leishmaniasis/human immunodeficiency virus co-infection in an endemic area in Northeast Brazil. Rev Soc Bras Med Trop. 54 https://doi.org/10.1590/0037-8682-0795-2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable for this submission.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding
Open access funding provided by HEAL-Link Greece.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kantzanou, M., Karalexi, M.A., Theodoridou, K. et al. Prevalence of visceral leishmaniasis among people with HIV: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 42, 1–12 (2023). https://doi.org/10.1007/s10096-022-04530-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-022-04530-4