Abstract
Bloodstream infection (BSI) is a common complication after living-donor liver transplantation (LDLT). Some patients develop recurrent BSIs. We evaluated the impacts of early recurrent BSIs (ER-BSIs) on outcomes in LDLT recipients. LDLT cases between 2008 and 2016 were included. Early BSI (E-BSI) was defined as a BSI event that occurred within 2 months after LDLT. ER-BSIs were defined as new-onset BSIs within 2 months due to another pathogen at a ≥ 48-h interval or a relapse of BSIs by the same pathogen at a ≥ 1-week interval, with negative cultures in between. The primary objective was evaluating the all-cause mortality of each group of LDLT recipients (90 days and 1 year). The secondary objectives were analyzing associated factors of each all-cause mortality and risk factors for early single BSI and ER-BSI. Among 727 LDLT recipients, 108 patients experienced 149 events of E-BSI with 170 isolated pathogens. Twenty-eight patients (25.9%, 28/108) experienced ER-BSI. The 1-year survival rates of patients without BSI, with early single BSI event, and with ER-BSIs were 92.4%, 81.3%, and 28.6%, respectively. ER-BSI was the most significant risk factor for 1-year mortality (adjusted HR = 5.31; 95% CI = 2.27–12.40). Intra-abdominal and/or biliary complications and early allograft dysfunction were risk factors for both early single BSI and ER-BSI. Interestingly, longer cold ischemic time and recipient operative time were associated with ER-BSI. LDLT recipients with ER-BSI showed very low survival rates accompanied by intra-abdominal complications. Clinicians should prevent BSI recurrence by being aware of intra-abdominal complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful management of infectious diseases is key to improving the prognosis of solid organ transplantation [1]. Infections are the most common cause of mortality during the early postoperative period [2, 3]. Bloodstream infections (BSIs), which typically occur early in the period of post-transplantation, are difficult to predict and prevent [1, 4]. Mortality in liver transplant (LT) patients who experience BSI events is 10–52% higher than in patients who do not experience BSI events, and the BSI-associated mortality of LT recipients tends to be higher than those of other transplant recipients (3–33% in heart, 2.5–11% in kidney, and 6–25% in lung transplantation) [4].
Prior studies have reported that 16.7–49.0% of LT recipients experienced at least one BSI, and more than half of BSI events occurred within the early postoperative period (30–100 days after LT) [5,6,7,8,9,10,11,12,13,14,15,16]. In other studies, 9.5–49.1% of patients experienced more than two BSI events due to the same or another pathogen [8,9,10, 12,13,14,15,16]. Considering that repetitive exposure to antibiotics is associated with drug toxicity and effects on human intestinal microbiota [1, 17, 18], patients with recurrent BSI events might have a different clinical prognosis compared with patients without BSI or single BSI. However, to the best of our knowledge, no data regarding the impact of recurrent BSI on outcomes in LT recipients have been reported.
As more than 70% of liver grafts were obtained from living donors in Korea (https://www.konos.go.kr), we conducted a retrospective cohort study to investigate the clinical outcome of living-donor liver transplant (LDLT) recipients with early recurrent BSIs (ER-BSIs).
Patients and methods
Study population and study periods
Adult LDLT recipients (≥ 18 years old) who underwent LT between January 2008 and December 2016 at Samsung Medical Center were collected retrospectively. Samsung Medical Center is a 1950-bed tertiary care referral hospital in Seoul, South Korea. Electronic medical records were reviewed, and the beginning of event observation was based on the day of the first liver transplant surgery. All patients were observed for 1 year or until death or loss to follow-up.
Definitions and study outcomes
True BSI was defined when bacteria or fungi were cultured from more than two separate blood samples or from a single blood sample in patients with concomitant clinical symptoms and focuses other than skin commensals [19]. Early BSIs (E-BSIs) were defined as BSI events within 2 months (60 days) after LDLT [7]. ER-BSIs were defined as new-onset BSIs within 2 months due to another pathogen at a ≥ 48-h interval or a relapse of BSIs by the same pathogen at a ≥ 1-week interval, with negative cultures in between, which was modified from the method used in a prior study [10]. Using these definitions, patients were placed into three groups: LDLT recipients without E-BSI, those with early single BSI, and patients with ER-BSI.
The following data were collected from electronic medical records: underlying liver disease (hepatitis B virus [HBV] infection, hepatitis C virus [HCV] infection, alcoholic liver disease, autoimmune liver disease, toxic hepatitis, hepatocellular carcinoma [HCC]), recipient and donor age, recipient and donor sex, recipient and donor blood type, recipient body mass index (BMI), Child-Pugh score, model for end-stage liver disease (MELD) score, presence of hepatorenal syndrome (HRS), previous history of spontaneous bacterial peritonitis, underlying diabetes mellitus (DM), hypertension, pre-transplant intensive care unit (ICU) stay, post-transplant ICU stay, early allograft dysfunction (EAD) defined as previous study (one or more of the following variables were present: bilirubin ≥ 10 mg/dL on postoperative day 7, INR ≥ 1.6 on postoperative day 7, and aminotransferase level > 2000 IU/mL within the first 7 postoperative days) [20], intra-abdominal complications and/or biliary complications (IABCs) within 2 months of the postoperative period, and acute rejection requiring immune modulation (such as steroid pulse therapy or change to other immunosuppressive agents) within 1 year of the postoperative period. IABCs were defined as peritonitis, complicated intra-abdominal fluid collection, biliary tract infection, and obstructive jaundice due to biliary stricture. If available, intraoperative data regarding donor anesthetic time, donor operation time, cold ischemic time (CIT), warm ischemic time, recipient anesthetic time, and recipient operation time were collected. In addition, data of appropriate antimicrobial use and source control at the first BSI event were collected. Empirical antimicrobial agents were considered appropriate if susceptible antimicrobial agents by in vitro susceptibility test result were administered within 48 h after obtaining the blood culture sample [21].
The primary objective of this study was evaluating the all-cause mortality of LDLT recipients without E-BSI, those with early single BSI, and those with ER-BSI (90 days and 1 year). The secondary objectives were analyzing associated factors of each all-cause mortality and risk factors for early single BSI and ER-BSI. Because intraoperative data might introduce confounding factors into the analysis for associated factors of mortality, these data were only included in analyzing risk factors for early single BSI and ER-BSI.
Immunosuppression for recipients
Tacrolimus, steroids, and mycophenolate mofetil (MMF) were the primary immunosuppressive therapies prescribed after LT. Until postoperative day (POD) 2, 500 mg of intravenous methylprednisolone was administered to all LT recipients and then tapered. On POD 3, tacrolimus was initiated. Target tacrolimus trough level was 10–15 ng/mL during the first month and 5–10 ng/mL thereafter. MMF was started on POD 1 at 750 mg twice a day. For ABO-incompatible LDLT recipients, plasma exchange and intravenous administration of a single dose of rituximab (375 mg/m2 body surface area) were implemented before transplantation, as described in our prior study [22].
Infection prevention and control
Cefotaxime and ampicillin/sulbactam were given until POD 2 if there was no evidence of colonization of multidrug-resistant (MDR) bacteria. An oral itraconazole solution (100 mg) was administered daily until POD 30. A daily single dose of trimethoprim/sulfamethoxazole was given postoperatively for 6 months. All recipients with HBV infection were treated with intravenous hepatitis B immunoglobulin (HBIG, Green Cross Corp, Yongin, Korea) and oral entecavir or tenofovir.
Blood culture and drug sensitivity test
All blood samples for cultures were obtained through peripheral veins and/or a central line. A Bactec-9240 system (Becton Dickinson, Sparks, MD, US) or a BacT/Alert 3D system (bioMérieux Inc., Marcy l’Etoile, France) was used for blood cultures. A Vitek II automated system (bioMérieux Inc.) was used to identify microbes and their antimicrobial agent sensitivities, with a standard identification card and the modified broth microdilution method. Blood isolates of methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus (VRE), extended-spectrum β lactamase (ESBL)–producing pathogens, and carbapenem-resistant Gram-negative bacilli (CRGNB) were defined according to the Clinical and Laboratory Standards Institute guidelines for 2017 [23].
Statistical analyses
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 25.0 for Windows (IBM Corp., 2018, Chicago, IL, US). To evaluate mortality of LDLT recipients in each group (LDLT recipients without BSI, with early single event of BSI, and with ER-BSI), the time-to-death and survival rates of LDLT recipients were analyzed using Kaplan-Meier curves. Adjustment of any factors associated with mortality was carried out by the Cox proportional hazard model. Variables with P < 0.10 in the univariate analysis were included in the multivariable analysis.
To evaluate factors associated with E-BSI or ER-BSI in LDLT recipients, a Student’s t test or Mann-Whitney U test was used to compare continuous variables, and the Chi-square test or Fisher’s exact test was used to compare categorical variables for identification of associated factors. The cutoff values for continuous variables were defined using the receiver operating characteristic curve with Youden-index. Variables with P < 0.10 in the univariate analysis along with variables considered potential clinically meaningful were included in the binary logistic regression model. All P values were two-tailed, and P values < 0.05 were considered to be statistically significant.
Results
Patient population and microbiology
We enrolled 727 patients in this study. Among these patients, 108 (14.8%, 108/727) experienced 149 events of E-BSI with 170 isolated pathogens. Twenty-eight (25.9%) of 108 patients with E-BSI experienced recurrent BSI (Fig. 1). Cases of LT recipients with ER-BSI are summarized in Supplementary Table S1.
Figure 2 shows the bacterial and fungal isolates from each episode of E-BSIs and its infection focus. While Gram-positive cocci (GPC) accounted for more than half of isolates from the first episodes of E-BSI, Gram-negative bacilli (GNB) were more frequently isolated than GPC from the recurrent BSI episodes (Fig. 2a). Enterococcus species were the most common isolates both in the initial episode and in recurrent BSI (second to the fourth episodes). Vancomycin resistance rates of Enterococcus isolates were significantly higher in recurrent BSIs (first vs. recurrent episodes: 16.7% vs. 56.3%, P = 0.001). Intra-abdominal infections (IAIs) were the most common focus of infections in the first episode and were significantly more common in recurrent episodes (59.3% vs. 82.9%, P = 0.007). Catheter-related infections were the second most common focus in E-BSI (Fig. 2b). The first BSI episodes had a median occurrence of POD 10 in patients with single E-BSI and of POD 8.5 in patients with ER-BSI, the difference between which was not statistically significant (P = 0.343).
Pathogens and foci of early bloodstream infections (BSIs) in living-donor liver transplant recipients. a Pathogens in first BSI events and ≥ 2 BSI event. b Foci in first BSI and ≥ 2 BSI events. a a5 isolates were non-C. albicans. bAll four isolates were non-C. albicans; GNB Gram-negative bacilli, ESBL-PE extended spectrum beta-lactamase producing Enterobacteriaceae, CRE carbapenem-resistant Enterobacteriaceae, CRAB carbapenem-resistant A. baumannii, CRPA carbapenem-resistant P. aeruginosa, CoNS coagulase-negative staphylococcus, GPC Gram-positive cocci, MRSA methicillin-resistant S. aureus, VRE vancomycin-resistant Enterococcus. b HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia, IAI intra-abdominal infection
All-cause mortality
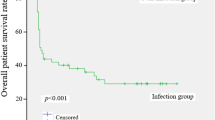
Mortality for LDLT recipients without E-BSI, with early single BSI, and with ER-BSI showed significantly lower survival rates with increasing episodes of BSI during the 1-year follow-up period (P < 0.001; Fig. 3). The 90-day all-cause mortalities of each group were 2.9%, 12.5%, and 50.0% (P < 0.001), and the 1-year all-cause mortalities of each group were 7.6%, 18.7%, and 71.5% (P < 0.001), respectively. There were 82 (11.3%) deaths in LDLT recipients during our 1-year observation period. Within 1 week before death, 43 (52.4%) patients experienced a total of 46 clinically documented infections. Intra-abdominal infections (31/46, 67.4%) were most common in these patients.
Associated factors of all-cause mortality
Univariate analysis revealed that isolation of MDR pathogens (MRSA, VRE, ESBL-producing Enterobacteriaceae, and CRGNB), hepatorenal syndrome, pre-transplant ICU stay, post-transplant ICU stay ≥ 10 days, EAD, and IABCs were associated with both 90-day and 1-year all-cause mortalities. HBV infection was associated with lower mortality (Supplementary Table S2).
Table 1 shows that ER-BSI had the highest hazard ratio in both 90-day and 1-year all-cause mortalities (adjusted hazard ratio [aHR] = 15.03, 95% confidence interval [CI] = 7.17–31.52 for 90-day all-cause mortality; aHR = 5.31, 95% CI = 2.27–12.40 for 1-year all-cause mortality). Pre-transplant ICU stay, IABC, DM, and acute rejection were associated with 1-year all-cause mortality. HBV infection was associated with a decrease in both all-cause mortalities.
Factors associated with early single BSI and ER-BSI
Associated clinical factors of LDLT recipients with early single BSI were compared with those of LDLT recipients without E-BSI. In the univariate analysis, old recipient age, low recipient BMI, high MELD score ≥ 18, HRS, pre-transplant ICU stay, post-transplant ICU stay ≥ 10 days, EAD, longer recipient operation time, and IABC were positively associated with early single BSI (Supplementary Table S3). In multivariable analysis, IABC showed the highest odds ratio for E-BSI (adjusted odds ratios [aOR] = 4.98, 95% CI = 2.97–8.32, P < 0.001). In addition, old age, MELD score ≥ 18, EAD, and post-transplant ICU stay ≥ 10 days were independently associated with E-BSI (Table 2).
Associated clinical factors of LDLT recipients with ER-BSI were compared with those of LDLT recipients without E-BSI. Upon univariate analysis, HCC, HRS, pre-transplant ICU stay, post-transplant ICU stay ≥ 10 days, longer donor operation time, longer CIT, longer recipient operation time, and IABC were associated with ER-BSI. (Supplementary Table S4). In multivariable analysis, IABC showed the highest OR for ER-BSI (aOR = 40.07, 95% CI = 8.93–179.82, P = 0.029). In addition, pre-transplant ICU stay, EAD, IABC, CIT ≥ 89 min, and recipient operation time ≥ 625 min were independently associated with ER-BSI (Table 2).
Meanwhile, LT recipients with early single BSI received more appropriate empirical antibiotics than those with ER-BSI (55.0% versus 39.3%); however, there was no statistical significance (P = 0.152, not shown in the table).
Discussion
This study confirmed that BSIs were very common and associated with higher mortality in LT recipients. We found that 14.8% of LDLT recipients experienced E-BSI events and 25.9% of those with E-BSI experienced recurrent BSIs. These results are similar to the findings of prior studies, which reported that 16.7–49.0% of LT recipients experienced BSI events at least once and more than half of BSI events occurred within the early postoperative period [4,5,6,7,8,9,10,11,12,13,14,15,16].
Poor outcomes of patients with BSIs consistently have been reported. In prior studies, BSI-associated mortality ranged from 10 to 52% in LT recipients [5,6,7,8,9,10,11,12,13,14,15,16]. Although the 90-day mortality rate was 2.9% in all LDLT recipients, this was higher in LDLT recipients with E-BSI (12.5%) and much higher in LDLT recipients with ER-BSI (50%) in this study. In addition, LDLT recipients with ER-BSIs had significantly higher 1-year attributable mortality than LDLT recipients with a single E-BSI (63.8% vs. 11.1%).
Numerous factors predicting outcomes for LT recipients have been reported. In addition to age, malnutrition, sarcopenia, MELD, delta MELD, presentation with acute hepatic failure, and infections were reported as risk factors for mortality in LT recipients [24,25,26,27,28]. However, the impact of BSIs on patient mortality has not been evaluated independently of other factors. In this study, previous ICU stay, IABC, and acute cellular rejection were risk factors for mortality. Although IABC was an associated factor with E-BSIs and ER-BSIs, ER-BSIs were associated with 90-day and 1-year mortality independent of IABC, showing the highest association. Since one-quarter of the 1-year all-cause mortality in our study population occurred in LDLT recipients with ER-BSI, who accounted for only 3.9% of the population, more efforts are needed to improve the outcomes of LDLT recipients with IABC who develop ER-BSI.
Prior studies have reported a variety of risk factors for BSI in LT recipients: old age, DM, hypoalbuminemia, prolonged ICU stay, high MELD score, and IABC [9,10,11, 13,14,15,16]. In contrast with previous studies, we focused on early-onset BSI and ER-BSI. To the best of our knowledge, no study has evaluated the factors associated with early single BSI and ER-BSI. As shown in prior studies, IABC was the most important risk factor for BSI in LT recipients in our study [9, 14, 16]. The strength of this association was even higher in ER-BSI than in early single BSI. Decompensated liver function and pre-transplant or prolonged postoperative ICU stay were reported as risk factors for early single BSI and ER-BSI in agreement with the findings of prior studies. In addition to patient characteristics, this is the first study that demonstrated an association between intraoperative parameters (prolonged CIT and recipient operation time) and BSI in LT recipients. Several studies have suggested that prolonged ischemic time or operative time is associated with poor outcomes of transplantation. Prolonged CIT has been reported as a risk factor for prolonged hospitalization for LT recipients [29]. In addition, it was identified as a risk factor of biliary stricture in duct-to-duct anastomosis for LDLT recipients. The study suggested that smaller size of grafts, hepatic arteries, and bile ducts in LDLT increase recipient vulnerability to prolonged CIT [30]. Given the factors mentioned above, prolonged CIT could lead to biliary duct injury and induce postoperative IABC followed by BSI. Prolonged operation time has been related to surgical site infections in any surgical intervention [31]. In our study, LDLT recipients with ER-BSI experienced prolonged donor and recipient operation time compared with those without E-BSI. Prolonged operation time might be associated with difficult anatomical variations in the recovery of donor liver and transplant to the recipient and could result in sustained and unsolved IABC in LDLT recipients to induce recurrent BSIs in LT recipients. Therefore, selecting appropriate donor grafts and decreasing ischemic and operation times might be critical to decrease ER-BSIs and to improve the clinical outcome in LDLT recipients.
There were several limitations to our study. First, the data cannot be generalized because this is a single-center study. The epidemiology could be different in another setting. Second, we included only LDLT recipients with E-BSI. The impact of BSIs could be different in deceased donor LT recipients. Moreover, late-onset BSI in LT recipients showed quite different from E-BSI in epidemiology and characteristics in a prior study [7]. Further studies are needed for deceased donor LT recipients and for evaluating the clinical effects of late-onset BSI.
In conclusion, ER-BSI predicted a very poor outcome for LDLT recipients and was closely related to emergence of IABCs. Graft preservation and operative time for transplantation were significantly associated with incidence of ER-BSI. To improve clinical outcomes and reduce BSIs in LDLT recipients, careful selection of donor livers and efforts to decrease CIT and operative time will be needed. Clinicians should try to prevent recurrence of BSI by focusing on intra-abdominal complications to improve clinical outcomes of LDLT recipients.
References
Fishman JA (2017) Infection in organ transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 17:856–879
Wagener G, Raffel B, Young AT, Minhaz M, Emond J (2013) Predicting early allograft failure and mortality after liver transplantation: the role of the postoperative model for end-stage liver disease score. Liver Transpl 19:534–542
Lee N, Kim JM, Kwon CHD et al (2014) Pre-transplant predictors for 3-month mortality after living donor liver transplantation. J Korean Soc Transplant 28:226–235
Kritikos A, Manuel O (2016) Bloodstream infections after solid-organ transplantation. Virulence. 7:329–340
Santos CA, Hotchkiss RS, Chapman WC, Olsen MA (2016) Epidemiology of bloodstream infections in a multicenter retrospective cohort of liver transplant recipients. Trans Direct 2:e67
Avkan-Oguz V, Ozkardesler S, Unek T et al (2013) Risk factors for early bacterial infections in liver transplantation. Transplant Proc 45:993–997
Lee SO, Kang SH, Abdel-Massih RC, Brown RA, Razonable RR (2011) Spectrum of early-onset and late-onset bacteremias after liver transplantation: implications for management. Liver Transpl 17:733–741
Iida T, Kaido T, Yagi S et al (2010) Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl 16:1379–1385
Bert F, Larroque B, Paugam-Burtz C et al (2010) Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl 16:393–401
Hashimoto M, Sugawara Y, Tamura S et al (2008) Bloodstream infection after living donor liver transplantation. Scand J Infect Dis 40:509–516
Bellier C, Bert F, Durand F et al (2008) Risk factors for Enterobacteriaceae bacteremia after liver transplantation. Transplant Int 21:755–763
Sganga G, Spanu T, Bianco G et al (2012) Bacterial bloodstream infections in liver transplantation: etiologic agents and antimicrobial susceptibility profiles. Transplant Proc 44:1973–1976
Kim SI, Kim YJ, Jun YH et al (2009) Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J 50:112–121
Shi SH, Kong HS, Xu J et al (2009) Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transplant Infect Dis 11:405–412
Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR (2000) Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl 6:54–61
Kim HK, Park YK, Wang HJ et al (2013) Epidemiology and clinical features of post-transplant bloodstream infection: an analysis of 222 consecutive liver transplant recipients. Infect Chemother 45:315–324
Jernberg C, Lofmark S, Edlund C, Jansson JK (2010) Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 156:3216–3223
Yilmaz C, Ozcengiz G (2017) Antibiotics: pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem Pharmacol 133:43–62
Seifert H (2009) The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clin Infect Dis 48(Suppl 4):S238–S245
Olthoff KM, Kulik L, Samstein B et al (2010) Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Trans 16:943–949
Lee CC, Lee CH, Hong MY, Tang HJ, Ko WC (2017) Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit Care 21:119
Kim JM, Kwon CH, Joh JW et al (2013) ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol 59:1215–1222
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 27th. Clinical and Laboratory Standards, Wayne, 2017
Gil E, Kim JM, Jeon K et al (2018) Recipient age and mortality after liver transplantation: a population-based cohort study. Transplantation. 102:2025–2032
Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR (2010) Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 10:1420–1427
Burroughs AK, Sabin CA, Rolles K et al (2006) 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 367:225–232
Kalafateli M, Mantzoukis K, Choi Yau Y et al (2017) Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle 8:113–121
Zakareya T, Abbasy M, Abdel-Razek W et al (2017) Utility of post-liver transplantation MELD and delta MELD in predicting early and late mortality. Eur J Gastroenterol Hepatol 29:1424–1427
Pan ET, Yoeli D, Galvan NTN et al (2018) Cold ischemia time is an important risk factor for post-liver transplant prolonged length of stay. Liver Trans 24:762–768
Park JB, Kwon CH, Choi GS et al (2008) Prolonged cold ischemic time is a risk factor for biliary strictures in duct-to-duct biliary reconstruction in living donor liver transplantation. Transplantation. 86:1536–1542
Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P (2017) Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect 18:722–735
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
The study was approved by the local ethical research committee (IRB number: 2019–06-001).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Kim, SH., Mun, S.J., Ko, JH. et al. Poor outcomes of early recurrent post-transplant bloodstream infection in living-donor liver transplant recipients. Eur J Clin Microbiol Infect Dis 40, 771–778 (2021). https://doi.org/10.1007/s10096-020-04074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-04074-5