Abstract
This study was designed to investigate the effect of low-temperature laminar flow ward (LTLFW) on the Acinetobacter baumannii pneumonia (MDR-ABP) in neurosurgical intensive care unit (NICU) patients. We evaluated whether patients in a LTLFW had significantly improved clinical outcomes as compared to those in nonconstant-temperature NICU (room temperature). The association of temperature with the prevalence of ABP and A. baumannii isolates (ABI) found in NICU patients was specifically investigated. In vitro microbiological experiments were conducted to measure the proliferation, antibiotic sensitivity, and genomic profiles of A. baumannii (AB) that grew in variable temperatures. MDR-ABP patients in LTLFW had significantly improved outcomes than those in the room temperature NICU. In addition, the numbers of ABI were positively associated with mean ambient outdoor temperatures (P = 0.002), with the incidence of ABP and average numbers of ABI among NICU patients being substantially lower in the winter as compared to other seasons. However, there were no significant seasonal variations in the other strains of the top five bacteria. Consistent with these clinical observations, AB growing at 20°C and 25°C had significantly reduced viability and antibiotic resistance compared to those growing at 35°C. The expression of genes related to AB survival ability, drug resistance, and virulence also differed between AB growing at 20°C and those at 35°C. LTLFW is effective in promoting the recovery of MDR-ABP patients because low temperatures reduced the density and virulence of AB and enhanced the efficacy of antibiotics, likely at the genetic level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii (AB) is a major cause of nosocomial infection of patients in intensive care units (ICU) and often develops resistance to antimicrobials [1]. A. baumannii pneumonia (ABP) is a significant risk for the mortality of patients in ICU [2, 3]. However, an optimal therapy for the treatment of multidrug resistance (MDR) ABP remains unclear [4].
Most respiratory infections such as influenza viruses and pneumococcus are more prevalent during the winter, and their outbreaks have been inversely correlated with environmental temperatures [5, 6]. However, several studies have shown that hospital-acquired infections with Gram-negative bacteria (GNB), especially AB, but not those with Gram-positive bacteria, are more common in the summer and are associated with high ambient outdoor temperatures (AOTs) [7,8,9,10]. The underlying mechanism for this seasonal variation remains poorly understood [10]. Studies reported in the literature are limited to the ward-based continuous surveillance, which could not determine whether A. baumannii isolates (ABI) are infected or colonized. Information on patient-specific analysis is scarce, and the independent association between bacterial infections and temperature is inconclusive, primarily due to the presence of considerable confounding variables [5, 8]. These reports also fail to provide practical temperature-related guidance for improving clinical outcomes of ICU patients.
We have conducted a retrospective clinical study and a microbiological trial to evaluate the impact of low-temperature laminar flow ward (LTLFW) and temperature variations on AB infections of patients admitted to NICU. To mechanistically understand this temperature-influenced AB infections, we also conducted in vitro experiments to characterize the impact of temperature on biological characteristics of AB.
Materials and methods
Patients
The retrospective collection of data from patients and their analyses have been approved by the ethics committee of Tianjin Medical University General Hospital (TMUGH), approval number IRB2018-YX-128. This study included patients admitted to NICU of TMUGH from 1 January 2013 to 31 December 2017.
We collected the information on demographics (sex and age) and primary diseases (e.g., intracerebral hematoma, traumatic brain injury). Patient’s conditions before ABP diagnosis and treatment situation, including Glasgow coma scale on admission, last white blood cell (WBC) counts before ABP diagnosis, surgeries and mechanical ventilation, types and duration of antibiotics (e.g., meropenem, cefoperazone/sulbactam, tigecycline), hospital stays prior to ABP diagnosis, NICU stays, and total hospital stays of the patients, were also included in the study through medical chart review. The recovery of MDR-ABP was defined as the primary outcome measure. And the secondary outcome variables for this study included last WBC before hospital discharge, the overall prognosis at hospital discharge, and in-hospital mortality.
For patients who developed multiple ABPs, we included only the first episode. To control for the total number of patient-days as a confounding variable, monthly or seasonal bacterial isolates and new cases of ABP were calculated as per 500 or 1500 patient-days, respectively. The date of diagnosis was defined as the time of first positive bacterial culture. MDR in AB was defined as resistance to three or more of the following five classes of antibiotics that are typically prescribed to treat AB: antipseudomonal fluoroquinolones, antipseudomonal cephalosporins, antipseudomonal carbapenems, β-lactam–β-lactamase inhibitor combinations, and aminoglycosides [11, 12]. All patients were continuously monitored until hospital discharge or death.
The inclusion criteria for ABP (diagnostic criteria) were as follows: (1) one or more positive respiratory secretion culture (sputum, tracheal aspirate, or broncho-alveolar lavage [BAL]) for AB according to the positive criteria defined in the next paragraph; (2) acute pulmonary infiltrations on chest x-ray (new, progressive or persistent infiltrate or cavitation); (3) two or more of the following findings: fever > 38°C, leukocytosis (≥ 12,000 WBC/μL) or leukopenia (<4000 WBC/μL), altered mental status in the elderly with no identifiable causes; new-onset purulent sputum or change in sputum character; worsening pulmonary gas exchange; new onset or worsening cough or dyspnea or tachypnea; rales or bronchial breathing: and (4) infections that could not be attributed to other sources [2, 13, 14].
The respiratory secretions were quantitatively cultured to meet the following criteria for the diagnosis of ABP: bacterial counts ≥ 103 colony-forming units per milliliter (cfu/mL) in the protected specimen brush and/or 104 or 105 cfu/mL in a BAL fluid specimen or 106 cfu/mL in an tracheal aspirate [2, 13]. For the semiquantitative culture, the cfu/mL was determined by the four-quadrant method and classified as follows: 0 = no growth; 1+ = 102 cfu/mL; 2+ = 103 cfu/mL; 3+ = 104 cfu/mL; and 4+ = 106 cfu/mL [3, 15].
The exclusion criteria: Patients who received less than three days of anti-infective treatment were excluded. These patients did not receive effective anti-infective treatment due to death of acute cerebral hernia or discharging automatically from the hospital without medical advice.
Treatments
Upon ABP diagnosis, patients were immediately quarantined, and strict precautions were taken to prevent cross-transmission [16]. The empirical antimicrobial regimen was maintained or adjusted on the basis of microbiological culture results [2, 17]. The common antibiotics used for AB were meropenem (2 g/8 h), cefoperazone/sulbactam (3 g/8 h), and tigecycline (100 mg twice a day in the first 48 h and then at 50 mg twice a day) as previously reported [1, 18].
As part of the NICU, there is a three-bed LTLFW that admitted highly contagious patients with uncontrollable fever to control fever and prevent cross-infection after diagnosed as MDR-ABP. Due to the limited beds, only a portion of MDR-ABP patients were admitted in LTLFW. LTLFW was equipped with an independent temperature control system that maintained the room temperature at 19°C, while the temperature of our regular NICU is non-constant which fluctuated from 22°C to 30°C with the seasons. The patients included in this study were divided into two groups: those who were treated in regular NICU during their hospital stay (non-LTLFW group) and those who were transferred from regular NICU to LTLFW (LTLFW group). Patients transferred to LTLFW were still treated by the team of doctors who previously managed them. For these patients in the NICU, every three patients were managed by a nurse and all patients share other types of staff. Standard precautions and strict hand hygiene practices were executed by all the staff.
The clinical recovery of ABP patients was defined as simultaneous normalizations of highest body temperature (≤ 38°C), leukocyte count (≤ 10,000 WBC/μL), PaO2/FIO2 ratio (> 187), and no or 1+ in AB culture of respiratory secretion [3, 17].
Environmental temperature data
AOTs data were collected from the publicly database and presented as monthly averages in the city of Tianjin [19]. Four seasons were defined as the spring (March 1 to May 31), the summer (June 1 to August 31), the autumn (Sept. 1 to Nov. 30), and the winter (Dec. 1 to the end of Feb).
Bacterial isolates
Microbiological results were obtained from the patients recruited between January 2013 and December 2017. Clinical isolates were identified using the Vitek 2 Compact System (bio-Merieux, Marcy-L’Etoile, France). We analyzed the first isolate from the same body location of a given patient during his or her hospital stay, regardless of whether it was colonization or infection. Repeatedly isolated strains were excluded [20]. The percentage of AB in total bacterial isolates (PABTBI) were calculated to exclude outbreaks or screening, which might exaggerate the number of AB.
AB culture and antibiotic susceptibility
The AB strains of 62,000 and 63,165 were cultured and analyzed with Klebsiella pneumoniae 62,907–2 as the control strains. All strains were randomly selected from clinical isolates. The impact of temperatures on the antimicrobial susceptibility was measured using a standardized disk-diffusion method (Kirby-Bauer) following the guidelines of the Clinical and Laboratory Standards Institute [21]. Briefly, bacterial strains were diluted to a final density of 0.5 and 0.005 McFarland units with phosphate-buffered saline, plated on Columbia blood agar plates (bio-Merieux, Marcy-L’Etoile, France) and cultured for 18 h at 20°C, 25°C, 30°C, or 35°C to allow the formation of bacterial colonies. In parallel, the bacterial broth was evenly spread onto Mueller-Hinton agar plates (0.5 McFarland, bio-Merieux, Marcy-L’Etoile, France). A cefoperazone/sulbactam (SCF) disk (75/30 mg per disk) (Oxoid Ltd., Basingstoke, Hampshire, UK) was then placed in the center of an inoculated plate and incubated at 20°C, 25°C, 30°C, or 35°C for 18 h. The size of the inhibition zone surrounding a SCF disk was then measured.
Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
mRNAs for the genes encoding resistance–nodulation–division (RND) pump (adeB, adeJ, and adeG), outer membrane protein A (OmpA), biofilm-associated protein (Bap), biofilm synthesis gene (bfs), and Class 1 integron (intI1) were quantitatively amplified in bacteria grown at 20°C or 35°C using qRT-PCR. These factors were chosen because of their demonstrated roles in the viability, resistance, and virulence of AB [22, 23]. OmpA is a major AB virulence factor, essential for killing host cells, whereas both Bap and Bfs support the formation of biofilm, which protects the bacteria from host immune response. The formation of biofilms results in persistent and difficult-to-treat chronic infections [23]. RND pumps and intI1 play a crucial role in intrinsic and acquired antibiotic resistance and are also vital for exhausting biocides, dyes, detergents, and organic solvents from AB. Three RND pumps have been reported in AB: AdeABC, AdeIJK, and AdeFGH [22, 24]. In addition, Fsr (Ferric siderophore receptor), which has been shown by De Silva et al. to upregulation at low temperature, was used as the control gene [23]. Table S1 lists the primers used for qRT-PCR. The expression of target genes was normalized against 16SrRNA, which served as the baseline control, using the 2-ΔΔCт method. Total RNA was extracted using an RNA Purification Kit (Biomiga, San Diego, USA) according to the manufacturer’s instructions.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (25th–75th percentile) as indicated in each specific data set. Categorical variables were presented as frequencies and percentages [no (%)]. Categorical variables were compared between groups using the Pearson chi-square test or continuity-adjusted chi-square test. Shapiro–Wilk test was used to determine whether the data was normally distributed. Unpaired Student’s t test, one-way ANOVA analysis of variance with Bonferroni correction, Mann–Whitney U test, or Kruskal–Wallis H test were conducted for continuous variables according to data distribution. We also evaluated the effect of the LTLFW on the recovery of MDR-ABP (primary outcome) using multivariate logistic regression models (Stepwise) to adjust for confounding. The demographics, primary diseases, patient’s condition before ABP diagnosis, and treatment situation were included in the model. Pearson’s correlation and linear regression were used to estimate the association among monthly average AOTs and numbers of ABI. Data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and R (version 3.6.1). Statistically significant level was defined at a P value of < 0.05.
Results
LTLFW improved clinical outcomes of MDR-ABP patients
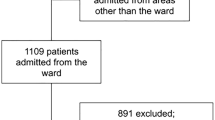
During the 5-year study period, a total of 170 suspected ABP patients were enrolled among the total of 2234 admissions in the 41,481 patient-days; only 11 other cases of AB infection (cerebrospinal fluid, bloodstream and wound) were documented. Among them, 26 patients were excluded as they were determined not to be AB infected. And the remaining 144 were diagnosed as ABP on the basis of clinical symptoms and microbiological tests, leading to an overall incidence of 6.45% or 3.47/1000 patient-days. And 99 patients of them were diagnosed as MDR-ABP (MDR rates, 68.75%), with an overall incidence of MDR-ABP being 4.43% or 2.39/1000 patient-days. Six MDR-ABP patients were excluded according to the exclusion criteria. Thirty patients with MDR-ABP were transferred from non-LTLFW to LTLFW wards, with the rest 63 MDR-ABP and 45 Non-MDR-ABP patients continued treated in non-LTLFW during their hospital stays (Fig. 1).
The clinical characteristics were comparable between the MDR-ABP patients in LTLFW and those in non-LTLFW groups. There was no statistical significance observed in terms of the demographics, primary diseases, patient’s condition before ABP diagnosis, and other treatment situations between the two groups (Table 1). Patients in LTLFW group had significantly improved outcomes as compared to those in non-LTLFW group, defined by high rates of recovery from MDR-ABP (73.33% vs. 50.79%, P = 0.039) and overall good prognosis (66.67% vs. 39.68%, P = 0.015), and by a low last WBC counts before hospital discharge (6.91 vs. 10.14, P = 0.045) or in-hospital mortality (10.00% vs. 31.75%, P = 0.044) on univariate analysis. Multivariate logistic regression analysis also revealed that LTLFW is effective in promoting the recovery of MDR-ABP (odds ratio, 2.8956 [95% confidence interval, 1.0513–8.6976], P = 0.462), after adjustment for the demographics, primary diseases, patient’s condition before ABP diagnosis, and other treatment situations (Table 2). The influence of other factors is very small compared with LTLFW.
Seasonal and temperature trends in ABI and ABP patients
A total of 2447 bacterial isolates were selected from 2013 to 2017. The top ten bacteria isolates were the Gram-positive Staphylococcus aureus and Staphylococcus epidermidis and the Gram-negative K. pneumoniae, AB, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Escherichia coli, Enterobacter cloacae, Enterobacter aerogenes, and Serratia marcescens. AB was the second most common and was identified as the pathogen in 401 (16.39%) of the 2447 strains. The monthly average total patient-days were 691.35 ± 89.98, which were relatively consistent during the entire study period (one-way ANOVA analysis, P = 0.334, Table S14). The average numbers of ABI among NICU patients were substantially lower in the winter months than in other seasons and were associated with mean AOTs (Fig. 2).
The average ABI were substantially low in the winter months and positively associated with mean AOTs. (a) The monthly average ABI (one-way ANOVA analysis, P < 0.001) and the PABTBI (one-way ANOVA analysis, P < 0.001) per 500 patient-days were substantially low in the winter months. (b) The average ABI in the winter were significantly lower than these in the other three seasons per 1500 patient-days, one-way ANOVA analysis, P = 0.001. Bonferroni test, ***P = 0.001, **P = 0.013, *P = 0.078. The PABTBI was also lower in the winter than in the other three seasons, One-way ANOVA analysis, P < 0.001. Bonferroni test, ***P < 0.001, **P = 0.001, *P = 0.021. (c, d) The monthly and seasonal average daily temperatures in the city of Tianjin. (e) The numbers of ABI were positively associated with monthly AOTs, y = 0.1328x + 3.0502, R2 = 0.6398, P = 0.002. ABI, A. baumannii isolates; AOTs, ambient outdoor temperatures; MABI, monthly A. baumannii isolates; MaxTemp, the average daily maximum temperatures; MeanTemp, the average daily temperatures; MinTemp, the average daily minimum temperatures; no, number; PABTBI, the percentage of AB in total bacterial isolates; SABI, seasonal A. baumannii isolates; °C, degree Celsius
There is no significant difference among the monthly or seasonal average of the total bacterial isolates (ATBI) from 2013 to 2017 (Fig. S1), but the average ABI and PABTBI were substantially lower in the winter months than in the rest seasons and increased during the summer, reaching a plateau in June (Fig. 2a, b). The highest monthly average ABI was in June, 4.73 times higher than the lowest in February (8.37 vs. 1.46; P < 0.001; Fig. 2a). Figure 2c and Fig. 2d show that the monthly and seasonal average daily temperatures in the city of Tianjin. There was a close correlation between monthly AOTs and monthly average numbers of ABI (Pearson’s r = 0.800, P = 0.002). Linear regression showed that temperature was linearly predictive of increasing numbers of ABI. The numbers of ABI were positively associated with monthly AOTs; there is a 4.35% increase in the monthly average ABI for each 1°C increase (Fig. 2e, R2 = 0.6398, P = 0.002). The numbers of new ABP cases in the summer were significantly higher than those in the winter and autumn per 1500 patient-days over the 5-year period (Fig. 3, P = 0.002).
The new ABP cases in summer were significantly higher than those in winter and autumn. One-way ANOVA analysis, P = 0.002. Bonferroni test, **P = 0.002, *P = 0.027. ABP, the new cases of ABP patients in each season; MeanTemp, the average daily temperatures of each season; no, number; °C, degree Celsius
However, there is no significant difference among the seasonal average of the other strains in the top five bacteria tested over the 5-year study period, including K. pneumoniae, S. aureus, P. aeruginosa, and S. maltophilia (Tables S2–S6).
Low-temperature reduced AB viability, virulence, and antibiotic resistance
K. pneumoniae, the top one bacterium tested during the 5-year study period, was selected as the control strain. As shown in Table 1, cefoperazone/sulbactam is the drug with the highest utilization rate in the treatment of multidrug resistance Acinetobacter baumannii pneumonia in our NICU. Therefore, cefoperazone/sulbactam was used for testing the impact of temperatures on antibiotic resistance. Consistent with the clinical observations, AB growing at 20°C and 25°C had a significantly reduced viability and were more sensitive to antibiotics as compared to those growing at 35°C (P < 0.001). There was no significant difference in AB growth at 30°C and 35°C (Fig. 4). We observed the upregulation of Fsr in AB cultured at 20°C compared to those at 35°C (P = 0.012). In contrast, the expression of genes encoding adeB, adeG, adeJ, OmpA, Bap, bfs, and intI1 were considerably reduced at 20°C (P < 0.005, Fig. 5). Together, these findings suggest that the vitality, antibiotic resistance, and virulence of AB are significantly reduced in the bacteria growing at 20°C.
AB growing at 20°C and 25°C had significantly reduced viability and antibiotic resistance than 35°C. (a, b) AB growing at 20°C and 25°C had obviously smaller and fewer colonies as compared to K. pneumoniae. (c, d) The zone diameter in 20°C and 25°C were obviously longer than 30°C and 35°C, one-way ANOVA analysis, P < 0.001. Bonferroni test, **20°C vs. 35°C (42.33 ± 0.58 vs. 31.33 ± 0.58, P < 0.001), 20°C vs. 25°C (42.33 ± 0.58 vs. 36.00 ± 1.00, P < 0.001), *25°C vs. 35°C (36.00 ± 1.00 vs. 31.33 ± 0.58, P < 0.001). AB 1, AB 62000; AB 2, AB 63165; KP, K. pneumoniae 62,907–2; SCF, Cefoperazone/Sulbactam disk; °C, degree Celsius
Relative expression of genes encoding adeB, adeG, adeJ, OmpA, Bap, bfs, intI1 and Fsr at 20°C and 35°C. ***P < 0.001, **P = <0.005, *P = 0.012. Bap, biofilm-associated protein; bfs, biofilm synthesis gene; intI1, Class 1 integron; Fsr, ferric siderophore receptor; OmpA, outer membrane protein A; °C, degree Celsius.
Discussion
We have conducted a study to define the influence of environmental temperature on clinical outcomes of MDR-ABP in NICU patients. This clinical study was synergized with a microbiological study of the temperature-related characteristics of AB bacteria. We made several key observations.
First, we demonstrated that patients with MDR-ABP treated in a LTLFW ward had a higher rate of recovery from MDR-ABP and overall good prognosis, resulting in significantly reduced in-hospital mortality (10.00%) as compared to those in regular NICU wards (31.75%). Our finding is also consistent with the previous reports that the crude mortality rates of patients with MDR-AB infections were 26%–47.8%, with 10%–43% attributable mortality [20, 25,26,27]. In addition, we found that the overall incidence of ABP (6.45%, 3.47/1000 patient-days) in our NICU was obviously lower than the incidences (17.00–27.63%, 4.18–14.26/1000 patient-days) have been reported [27,28,29]. The PABTBI of 16.39% was also lower than that of 19.1%–38.7% in other ICUs as previously reported [30, 31]. The improved outcomes were observed in LTLFW patients, and the reduced infectious rate suggests that LTLFW provides a more effective way to treat infections of ABP patients in NICU.
Second, we observed seasonal or temperature variations in the incidence of ABI and ABP in China, consistent with previous reports in other countries [32,33,34]. This may be the reason why AB infections are particularly common in the summer or tropical climates [12, 28, 35], where GNB, especially those environmentally acquired, proliferate faster and have enhanced virulence, increasing their colonization and infection [36,37,38,39]. High temperatures may also promote the formation of a biofilm “bloom” of Acinetobacter species [40]. Similar seasonal variations have also been reported for other GNB such as K. pneumoniae and E. coli [37, 38, 41,42,43], as compared to Gram-positive infections that are more common in the winter [9, 44, 45]. Interestingly, we observed the seasonal variations primarily for AB, not for other strains of the top five bacteria tested.
Third, the microbiological trial indicates that AB grew slowly and had reduced antibiotic resistance in low temperature environment (P < 0.001). Furthermore, the expression of genes related to vitality, virulence, and antibiotic resistance of AB is significantly reduced when they were cultured in 20°C compared to those grew at 35°C. De Silva et al. suggested that the upregulation of Fsr may be due to lower iron transport efficiency at low temperature [23]. This finding may explain why patients with MDR-ABP treated in LTLFW wards achieved significantly improved outcome. Owing to the ability to survive on environmental surfaces and patients skin, AB strains can cause widespread contamination and cross-infection in the hospital environment [12, 27]. An increased density of environmental AB will increase the probability of human’s exposure. The ability to invade human tissues may increase owing to the expression of virulence factors, and increased resistance makes it difficult to cure.
The limitation of this report is that this small sample size, and retrospective study was performed in a single-center, and thus, prospective randomized trials are required to determine the effect of temperature on AB infections. It is important to note that other climatic variables, such as humidity and precipitation that were not examined in this study may confound the correlations between AB infections and temperature.
Conclusions
We reported that substantially decreased AB infectious in the winter months and lower AOTs. LTLFW might be reasonable for promoting the recovery of MDR-ABP patients because a low-temperature environment reduces the density, virulence, and antibiotic resistance of AB at genetic levels. This finding is significant in improving the prognosis of patients with MDR-ABP.
Abbreviations
- AB:
-
Acinetobacter baumannii
- ABI:
-
Acinetobacter baumannii isolates
- ABP:
-
Acinetobacter baumannii pneumonia
- AOTs:
-
Ambient outdoor temperatures
- ATBI:
-
Average of the total bacterial isolates
- BAL:
-
Broncho-alveolar lavage
- Bap:
-
Biofilm-associated protein
- bfs:
-
Biofilm synthesis gene
- cfu/mL:
-
colony-forming units per milliliter
- Fsr:
-
Ferric siderophore receptor
- GCS:
-
Glasgow coma scale
- GNB:
-
Gram-negative bacteria
- ICH:
-
Intracerebral hematoma
- ICU:
-
Intensive care units
- intI1:
-
Class 1 integron
- LTLFW:
-
Low-temperature laminar flow ward
- MDR:
-
Multidrug resistance
- NICU:
-
Neurosurgical intensive care units
- OmpA:
-
Outer membrane protein A
- PABTBI:
-
The percentage of AB in total bacterial isolates
- qRT-PCR:
-
Quantitative real-time reverse transcriptase polymerase chain reaction
- RND:
-
Resistance–nodulation–division
- SCF:
-
Cefoperazone/Sulbactam disk
- TBI:
-
Traumatic brain injury
- TMUGH:
-
Tianjin Medical University General Hospital
References
Garnacho-Montero J, Dimopoulos G, Poulakou G, Akova M, Cisneros JM, De Waele J, Petrosillo N, Seifert H, Timsit JF, Vila J, Zahar JR, Bassetti M, European Society of Intensive Care M (2015) Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med 41(12):2057–2075. https://doi.org/10.1007/s00134-015-4079-4
Garnacho-Montero J, Ortiz-Leyba C, Fernandez-Hinojosa E, Aldabo-Pallas T, Cayuela A, Marquez-Vacaro JA, Garcia-Curiel A, Jimenez-Jimenez FJ (2005) Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 31(5):649–655. https://doi.org/10.1007/s00134-005-2598-0
Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ (2001) Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med 163(6):1371–1375. https://doi.org/10.1164/ajrccm.163.6.2007020
Jung SY, Lee SH, Lee SY, Yang S, Noh H, Chung EK, Lee JI (2017) Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care 21(1):319. https://doi.org/10.1186/s13054-017-1916-6
Dowell SF, Ho MS (2004) Seasonality of infectious diseases and severe acute respiratory syndrome–what we don't know can hurt us. Lancet Infect Dis 4(11):704–708. https://doi.org/10.1016/s1473-3099(04)01177-6
Dowell SF, Whitney CG, Wright C, Rose CE Jr, Schuchat A (2003) Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis 9(5):573–579
McDonald LC, Banerjee SN, Jarvis WR (1999) Seasonal variation of Acinetobacter infections: 1987-1996. Nosocomial infections surveillance system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 29(5):1133–1137. https://doi.org/10.1086/313441
Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN (2011) Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One 6(9):e25298. https://doi.org/10.1371/journal.pone.0025298
Perencevich EN, McGregor JC, Shardell M, Furuno JP, Harris AD, Morris JG, Fisman DN, Johnson JA (2015) Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol 29(12):1124–1131. https://doi.org/10.1086/592698
Richet H (2012) Seasonality in gram-negative and healthcare-associated infections. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 18(10):934–940. https://doi.org/10.1111/j.1469-0691.2012.03954.x
Paterson DL (2006) The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 43(Suppl 2):S43–S48. https://doi.org/10.1086/504476
Munoz-Price LS, Weinstein RA (2008) Acinetobacter infection. N Engl J Med 358(12):1271–1281. https://doi.org/10.1056/NEJMra070741
Yang YS, Lee YT, Huang TW, Sun JR, Kuo SC, Yang CH, Chen TL, Lin JC, Fung CP, Chang FY (2013) Acinetobacter baumannii nosocomial pneumonia: is the outcome more favorable in non-ventilated than ventilated patients? BMC Infect Dis 13:142. https://doi.org/10.1186/1471-2334-13-142
Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, Samaria JK, Gaur SN, Jindal SK, Pneumonia Guidelines Working G (2012) Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: joint ICS/NCCP(I) recommendations. Lung India : official organ of Indian Chest Society 29(Suppl 2):S27–S62. https://doi.org/10.4103/0970-2113.99248
Zeng J, Wang CT, Zhang FS, Qi F, Wang SF, Ma S, Wu TJ, Tian H, Tian ZT, Zhang SL, Qu Y, Liu LY, Li YZ, Cui S, Zhao HL, Du QS, Ma Z, Li CH, Li Y, Si M, Chu YF, Meng M, Ren HS, Zhang JC, Jiang JJ, Ding M, Wang YP (2016) Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med 42(6):1018–1028. https://doi.org/10.1007/s00134-016-4303-x
Ben-Chetrit E, Wiener-Well Y, Lesho E, Kopuit P, Broyer C, Bier L, Assous MV, Benenson S, Cohen MJ, McGann PT, Snesrud E, Levin PD (2018) An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: long-term impact for the ICU and hospital. Crit Care 22(1):319. https://doi.org/10.1186/s13054-018-2247-y
Reina R, Estenssoro E, Saenz G, Canales HS, Gonzalvo R, Vidal G, Martins G, Das Neves A, Santander O, Ramos C (2005) Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med 31(8):1058–1065. https://doi.org/10.1007/s00134-005-2691-4
Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y (2019) Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control 8:52. https://doi.org/10.1186/s13756-019-0502-x
The temperature in tianjin. https://www.tianqi.com/qiwen/city_tianjin/. Accessed December 2018
Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC (2007) Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 28(6):713–719. https://doi.org/10.1086/517954
CLSI (2018) Performance standards for antimicrobial susceptibility testing, vol CLSI supplement M100. 28th edn. Clinical and Laboratory Standards Institute,
Bazyleu A, Kumar A (2014) Incubation temperature, osmolarity, and salicylate affect the expression of resistance-nodulation-division efflux pumps and outer membrane porins in Acinetobacter baumannii ATCC19606T. FEMS Microbiol Lett 357(2):136–143. https://doi.org/10.1111/1574-6968.12530
De Silva PM, Chong P, Fernando DM, Westmacott G, Kumar A (2018) Effect of incubation temperature on antibiotic resistance and virulence factors of Acinetobacter baumannii ATCC 17978. Antimicrob Agents Chemother 62(1). https://doi.org/10.1128/AAC.01514-17
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21(3):538–582. https://doi.org/10.1128/CMR.00058-07
Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, Srinivasan A (2007) Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13(1):97–103. https://doi.org/10.3201/eid1301.060716
Falagas ME, Bliziotis IA, Siempos II (2006) Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care 10(2):R48. https://doi.org/10.1186/cc4869
Munier AL, Biard L, Legrand M, Rousseau C, Lafaurie M, Donay JL, Flicoteaux R, Mebazaa A, Mimoun M, Molina JM (2018) Incidence, risk factors and outcome of multi-drug resistant Acinetobacter baumannii nosocomial infections during an outbreak in a burn unit. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. https://doi.org/10.1016/j.ijid.2018.11.371
Chim H, Tan BH, Song C (2007) Five-year review of infections in a burn intensive care unit: high incidence of Acinetobacter baumannii in a tropical climate. Burns : journal of the International Society for Burn Injuries 33(8):1008–1014. https://doi.org/10.1016/j.burns.2007.03.003
Durdu B, Kritsotakis EI, Lee ACK, Torun P, Hakyemez IN, Gultepe B, Aslan T (2018) Temporal trends and patterns in antimicrobial-resistant gram-negative bacteria implicated in intensive care unit-acquired infections: a cohort-based surveillance study in Istanbul, Turkey. Journal of global antimicrobial resistance 14:190–196. https://doi.org/10.1016/j.jgar.2018.04.015
Tao L, Hu B, Rosenthal VD, Gao X, He L (2011) Device-associated infection rates in 398 intensive care units in Shanghai, China: international nosocomial infection control consortium (INICC) findings. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 15(11):e774–e780. https://doi.org/10.1016/j.ijid.2011.06.009
Chittawatanarat K, Jaipakdee W, Chotirosniramit N, Chandacham K, Jirapongcharoenlap T (2014) Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a northern Thai tertiary-care university based general surgical intensive care unit. Infection and drug resistance 7:203–210. https://doi.org/10.2147/idr.s67267
Caldeira SM, Cunha AR, Akazawa RT, Moreira RG, Souza Ldo R, Fortaleza CM (2015) Weather parameters and nosocomial bloodstream infection: a case-referent study. Rev Saude Publica 49(0):19. https://doi.org/10.1590/s0034-8910.2015049005438
Fournier PE, Richet H (2006) The epidemiology and control of Acinetobacter baumannii in health care facilities. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 42(5):692–699. https://doi.org/10.1086/500202
Kim YA, Kim JJ, Won DJ, Lee K (2018) Seasonal and temperature-associated increase in community-onset Acinetobacter baumannii Complex colonization or infection. Annals of laboratory medicine 38(3):266–270. https://doi.org/10.3343/alm.2018.38.3.266
Fortaleza CM, Caldeira SM, Moreira RG, Akazawa RT, Corrente JE, de Souza LR, da Cunha AR (2014) Tropical healthcare epidemiology: weather determinants of the etiology of bloodstream infections in a Brazilian hospital. Infect Control Hosp Epidemiol 35(1):85–88. https://doi.org/10.1086/674392
Raetz CR, Reynolds CM, Trent MS, Bishop RE (2007) Lipid a modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329. https://doi.org/10.1146/annurev.biochem.76.010307.145803
Kaier K, Frank U, Conrad A, Meyer E (2010) Seasonal and ascending trends in the incidence of carriage of extended-spectrum ss-lactamase-producing Escherichia coli and Klebsiella species in 2 German hospitals. Infect Control Hosp Epidemiol 31(11):1154–1159. https://doi.org/10.1086/656748
Anderson DJ, Richet H, Chen LF, Spelman DW, Hung YJ, Huang AT, Sexton DJ, Raoult D (2008) Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis 197(5):752–756. https://doi.org/10.1086/527486
Freeman JT, Anderson DJ, Sexton DJ (2009) Seasonal peaks in Escherichia coli infections: possible explanations and implications. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 15(10):951–953. https://doi.org/10.1111/j.1469-0691.2009.02866.x
Christie C, Mazon D, Hierholzer W Jr, Patterson JE (1995) Molecular heterogeneity of Acinetobacter baumanii isolates during seasonal increase in prevalence. Infect Control Hosp Epidemiol 16(10):590–594
Rodrigues FS, Clemente de Luca FA, Ribeiro da Cunha A, Fortaleza C (2018) Season, weather and predictors of healthcare-associated gram-negative bloodstream infections: a case-only study. The Journal of hospital infection. https://doi.org/10.1016/j.jhin.2018.06.015
Chazan B, Colodner R, Edelstein H, Raz R (2011) Seasonal variation in Escherichia coli bloodstream infections in northern Israel. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 17(6):851–854. https://doi.org/10.1111/j.1469-0691.2010.03327.x
Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM (2009) Seasonal variation in Escherichia coli bloodstream infection: a population-based study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 15(10):947–950. https://doi.org/10.1111/j.1469-0691.2009.02877.x
Goncalves-Pereira J, Povoa PR, Lobo C, Carneiro AH (2013) Bloodstream infections as a marker of community-acquired sepsis severity. Results from the Portuguese community-acquired sepsis study (SACiUCI study). Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 19(3):242–248. https://doi.org/10.1111/j.1469-0691.2012.03776.x
Schwab F, Gastmeier P, Meyer E (2014) The warmer the weather, the more gram-negative bacteria - impact of temperature on clinical isolates in intensive care units. PLoS One 9(3):e91105. https://doi.org/10.1371/journal.pone.0091105
Acknowledgments
The authors acknowledge the role of all support staff in the study.
Funding
This work was supported by the National Natural Science Foundation of China (No: 81671221 and 81271359); the Tianjin Research Program of Application Foundation and Advanced Technology of China (No: 14ZCZDSY00179), and the Clinical Study of Tianjin Medical University (No: 2017kylc007).
Author information
Authors and Affiliations
Contributions
RCJ, ZTG and HLL contributed substantially to the collection of literature, the study design, the data interpretation, and the writing of the manuscript. RCJ and HJY reviewed and revised the articles, and performed the quality assessment. JL, ZTG and ZDH performed microbiological trials. DQZ, XHL, CG, JHH, YQ and YMS performed data collection and statistical analyses. WQ, SA, YT, and JS performed the interpretation of data and study oversight. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors report no conflicts of interest relevant to this article.
Ethical approval
The retrospective collection of data from patients and their analyses have been approved by the ethics committee of Tianjin Medical University General Hospital (approval number: IRB2018-YX-128).
Statement of informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 761 kb)
Rights and permissions
About this article
Cite this article
Gong, Z., Li, J., Luo, H. et al. Low-temperature laminar flow ward for the treatment of multidrug resistance Acinetobacter baumannii pneumonia. Eur J Clin Microbiol Infect Dis 39, 877–887 (2020). https://doi.org/10.1007/s10096-019-03790-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03790-x