Abstract
To evaluate the effectiveness of trimetoprim-sulfametoxazole (TMP-SMX) for treatment of ventilator-associated pneumonia (VAP). A retrospective cohort study including patients with VAP from 2011 to 2017. Two groups were analysed: TMP-SMX group, including patients who had received TMP-SMX (as first-line and as de-escalation), and No-TMP-SMX group, including patients who had not received TMP-SMX treatment. Primary clinical outcome was mortality at 30 days from starting the antibiotic treatment (T30). Secondary outcomes were mortality at end of treatment (EoT), day survival at T30, and acquisition of multidrug-resistant bacteria during hospitalization in intensive care unit. Eighty cases of VAP were included and devised into two groups: No-TMP-SMX (31/80; 39%) and TMP-SMX (49/80; 61%). Univariate analysis showed no significant differences were found when the TMP-SMX group was compared with the No-TMP-SMX group, except for frequency of male gender (p = 0.025). No significant statistical correlations between mortality at T30 and individual factors were detected by the multivariate model. No cases of either severe allergy or Clostridium difficile disease were reported in the TMP-SMX and No-TMP-SMX groups. TMP-SMX treatment was not associated with higher mortality at EoT and T30 in comparison with the No-TMP-SMX group. TMP-SMX had a good safety profile, in terms of ecology (acquisition of MDR bacteria and Clostridium difficile disease) and clinical management (no allergy events).
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is defined as a nosocomial infection of pulmonary parenchyma which develops in patients who have been mechanically ventilated in the intensive care unit (ICU) for at least 48 h [1]. Epidemiology of VAP, however, differs according to the geographical area, in particular when it comes to the prevalence of multidrug-resistant (MDR) bacteria [2,3,4,5,6,7,8].
Trimetoprim-sulfametoxazole or co-trimoxazole (TMP-SMX) is a bactericidal association of two sulphonamides which is naturally active against a broad spectrum of microorganisms, including the most frequents agents of VAP, with the exception of Pseudomonas aeruginosa which is naturally resistant to TMP-SMX. It is approved for pneumonia (notably Pneumocystis jirovecii pneumonia and community-acquired pneumonia) [9], but it is not currently recommended for the empiric treatment of VAP by American or European guidelines, even though its use is not formally contraindicated in ventilator-associated respiratory infections [10, 11]. Moreover, TMP-SMX is already used in methicillin-resistant Staphylococcus aureus (MRSA)–associated VAP, as an alternative to vancomycin [12, 13].
The aim of this study was to retrospectively evaluate the effectiveness of TMP-SMX for treatment of VAP compared with TMP-SMX-free regimens.
Materials and methods
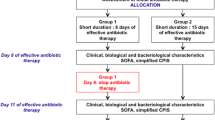
A retrospective cohort study was conducted in a hospital accounting for 350 acute care beds in the Ile de France region in France, from 1 January 2011 to 31 December 2017. Patients were considered eligible for the study when they had diagnosis of VAP, and they did not meet any of the following exclusion criteria: (i) absence of isolates in cultures from lower respiratory tract samples; (ii) positivity of cultures from lower respiratory tract samples for Pseudomonas spp. and other TMP-SMX naturally resistant bacteria.
This study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The local institutional review board did not require patients’ consent due to the retrospective character of the study. Because of this and along with the fact that this study did not require further laboratory analysis or different clinical management compared with daily clinical routine, a written consent form was not proposed to any eligible patient.
Diagnosis of VAP was made at ICU by the intensive care specialist according to the international definition of VAP [1]. A collegiate body, including intensive care specialists, clinical microbiologists and infectious diseases specialists, performed daily reviews of antibiotic treatments according to clinical evolution and microbiological results. De-escalation was carried out in accordance with international definitions [14, 15].
In the case of absence of microbial isolates at VAP onset, first-line treatment always included penicillin plus β-lactamase inhibitors (piperacillin/tazobactam or amoxicillin/clavulanic acid). De-escalation was proposed when microbiologic isolates became available after the starting of antibiotic treatment. Because the culture of lower respiratory tract samples was routinely performed during invasive ventilation whether or not there were clinical signs of VAP, some patients already had microbial isolates before the starting of antibiotic treatment. For these patients, a targeted antibiotic treatment was started as first-line treatment. For all molecules, the highest posology was prescribed and adapted to estimated glomerular filtration rate (eGFR) [16].
TMP-SMX was prescribed only when a susceptible isolate was identified either at VAP onset (targeted first-line treatment) or after beginning a No-TMP-SMX treatment (de-escalation). In case of TMP-SMX susceptibility, TMP-SMX was always preferred to other susceptible molecules, except if there were some contraindications (other concurrent infections caused by TMP-SMX-resistant bacteria, known history of TMP-SMX allergy, severe anaemia). A maximal dose of 160/800 mg tid or qid of TMP-SMX was prescribed and adapted to eGFR and patient’s weight [16].
Patients were devised into two groups: (i) TMP-SMX group, which included patients who received TMP-SMX as treatment for VAP, as first-line therapy or de-escalation; (ii) No-TMP-SMX group, which included patients who did not receive TMP-SMX as treatment for VAP.
Data about patients’ characteristics, laboratory analysis and treatment outcomes were collected from software used in daily clinical practice (Sillage v15.5.1.22 and CGM Lab channel 1.20.33686) and included the following: age, gender, body mass index (BMI), co-morbidities (diabetes, heart disease, lung disease, liver disease, solid or hematologic neoplasia, severe acute or chronic kidney disease), antibiotic treatment before the onset of VAP, simplified acute physiology score II (SAPS-II), early VAP, shock (at onset of VAP) and bacteria isolates from lower respiratory tract samples. The cutoff for the definition of severe kidney disease was eGFR < 30 ml/min [17]. Early VAP was defined when onset of VAP occurred within 96 h from the start of mechanical ventilation [18]. Patients were considered in shock when they needed vasopressors for maintaining a mean arterial pressure of at least 65 mmHg at the onset VAP symptoms [19].
The primary clinical outcome was mortality at 30 days after antibiotic treatment initiation (T30) while secondary clinical outcomes were as follows: (i) mortality at the end of treatment (EoT); (ii) day survival at T30, defined as the number of days of survival for each patient during the 30 days after antibiotic treatment initiation; (iii) acquisition of MDR bacteria during hospitalization in ICU. For definition of MDR bacteria acquisition, results of nasopharyngeal and rectal swabs (routinely obtained at admission and discharge) and other samples (obtained during the hospitalization according to the patient’s clinical evolution) were all considered.
For univariate analysis, χ2 test (qualitative variables) and Student’s t test (quantitative variables) were used. Quantitative variables were presented in the text as mean values.
Differences in the incidence of the four clinical outcomes were analysed. Moreover, differences at baseline were calculated for the following individual characteristics: age, gender, BMI, co-morbidities, SAPS-II, antibiotic treatment before VAP onset, early VAP, shock (at VAP onset), microbial isolates from lower respiratory tract samples, carriage of MDR bacteria (both at admission and discharge).
For multivariate analysis, a multiple logistic regression analysis was performed on the overall population to explore whether individual factors might explain differences in the primary clinical outcome (mortality at T30). Parameters included in multivariate analysis were chosen according to univariate analysis results (p ≤ 0.250). Moreover, the use of TMP-SMX was also included in the multivariate analysis to evaluate the impact of TMP-SMX on mortality at T30.
All analyses were performed using R, the language for statistical computing (Vienna, Austria; https://www.r-project.org/). Nominal statistical significance was set at p < 0.050.
Results
During the study period, a total of 126 cases of VAP were identified, and 80/126 (63.5%) were included in the study. According to exclusion criteria, 12/126 (9.5%) patients were excluded because no microbial isolate was identified from lower respiratory tract samples, and 34/126 (27%) patients were excluded because of Pseudomonas aeruginosa isolates identified in lower respiratory tract samples. Characteristics of the population included in the study are summarised in Table 1.
All patients without microbial isolates at VAP onset or with microbial isolates resistant to TMP-SMX were given monotherapy using a penicillin plus/minus penicillinase inhibitor while 6/80 (8%) were given TMP-SMX-free therapy using other molecules or associations. The remaining patients with bacterial isolates susceptible to TMP-SMX (28/80; 35%) were given monotherapy using TMP-SMX as first line, and none of them were switched towards another molecule. No patient started a multidrug regimen which included TMP-SMX as first line. A total of 36/80 (45%) patients were switched to another molecule. Among them, 21/80 (26.3%) patients were switched to TMP-SMX from a TMP-SMX-free regimen. More specifically, 20/21 (95.2%) switched to a TMP-SMX monotherapy while only 1/21 (4.8%) patient was switched to a combination therapy (TMP-SMX plus intravenous amikacin).
Antibiotic resistance to TMP-SMX was detected in 9/80 (11.3%) isolates. No therapy was discontinued (with or without TMP-SMX) for adverse events. No cases of severe allergy were reported in patients treated with TMP-SMX or with other molecules. No cases of Clostridium difficile disease (defined as the presence of binary toxin in stools) were reported in the overall population (in TMP-SMX-receiving and non-receiving patients).
When the TMP-SMX group and the No-TMP-SMX group were compared, no statistical differences were observed (Table 1), except for frequency of male gender (p = 0.025). No statistical differences between the two groups were observed concerning mortality rates at EoT and T30 (p = 0.874 and 0.581, respectively), day survival at T30 (p = 0.623) and rate of MDR bacteria acquisition (p = 0.108).
The multivariate model did not show any statistical correlation between mortality at T30 and other variables (male gender, BMI, SAPS-II, TMP-SMX, MDR bacteria acquisition), as illustrated in Table 2.
Discussion
To the best of our knowledge, this study analysed for the first time the effectiveness of TMP-SMX for the treatment of VAP in a real-life cohort.
In our study, effectiveness of treatment with TMP-SMX was not significantly different than treatment without TMP-SMX. Indeed, no differences were found in terms of clinical outcomes (mortality at T30 and EoT, day survival at T30 and MDR bacteria acquisition) when the No-TMP-SMX group was compared with the TMP-SMX group. This also included critically ill patients with high SAPS-II and septic shock at presentation.
The use of TMP-SMX as first-line therapy in VAP is limited by the lack of activity on Pseudomonas aeruginosa [9], one of the most frequent causes of VAP, which needs to be taken into consideration empirically treating VAP [10, 11]. Thus, we can suggest a larger use of TMP-SMX for VAP only when microbiological diagnosis and susceptibility tests are available, both as a first-line regimen and as adapted treatment (de-escalation). Basing TMP-SMX use on availability of susceptibility test makes this approach suitable also for settings with higher resistance rate than France. On the contrary, TMP-SMX in the empiric treatment of VAP should be avoided in any setting because the risk of TMP-SMX-resistant strains (notably Pseudomonas aeruginosa) among patients hospitalised in ICU is too high [20]. We consider that the results of our study are convincing enough to promote further studies and to be evaluated in a randomised clinical trial (RCT).
There are at least three reasons to encourage the use of TMP-SMX in VAP. First of all, TMP-SMX allows to spare β-lactams and fluoroquinolones and, consequently, to reduce pressure of selection and emergence of MDR bacteria, in particular, the extended spectrum β-lactamase and carbapenemase-producing gram-negative bacteria and Pseudomonas aeruginosa [21,22,23,24,25]. Secondly, TMP-SMX has an extremely high intestinal absorption and it reaches high concentration in lung tissue when it is administrated either orally or intravenously. This property can facilitate a quick switch from an intravenous to an oral regimen, at least in uncritical patients [26,27,28]. Finally, the spread of vancomycin-intermediate Staphylococcus aureus (VISA) is increasing all over the world and is associated with an increased risk of mortality [29,30,31]. Most unfortunately, the best treatment of VISA infection has not yet been established. TMP-SMX could be a valid alternative to vancomycin and it is cheaper than its principal competitors, linezolid and telavancin, even though there is a lack of data about TMP-SMX’s cost-effectiveness in treatment of healthcare-associated pneumonia, namely VAP [12, 13, 32,33,34]. Moreover, according to the World Health Organization, linezolid has to be considered as a reserve antibiotic that should be used as a last-resort option, while TMP-SMX is part of the access group (i.e. antibiotics which are supposed to be widely used in first-line treatments and reliably available in every setting) [35].
Concerning safety, no TMP-SMX-based treatment was discontinued because of safety issues, including severe allergy. These data are encouraging and could suggest that tolerability of TMP-SMX in ICU patients could be higher than patients with acquired immune deficiency syndrome, but this finding needs to be confirmed in further studies with larger populations [36].
This study presents several limitations: (i) because of study design (retrospective cohort study), a certain amount of missing data is predictable, even if all consecutive patients were included and computerised medical files were used for data collection. Variables with a high number of missing data were excluded from the logistic regression analysis to preserve the effectiveness of the statistical analysis; (ii) no power analysis and sample size calculation were estimated; (iii) the relatively small sample size may have limited the power of statistical analysis [37, 38]; (iv) data about clinical safety were not extensively collected which makes analysis of the safety profile of TMP-SMX limited; (iv) this was a single-centre study; thus, conclusions could not be directly applicable to other centres with significantly different populations.
In conclusion, this study showed that TMP-SMX treatment for VAP was not associated with higher rates of mortality at EoT and T30 than TMP-SMX non-including treatment. Moreover, TMP-SMX had a good safety profile, in terms of ecology (acquisition of MDR bacteria and Clostridium difficile disease) and clinical management (no allergy events). According to these results, the use of TMP-SMX could be enhanced in VAP in the case of microbiological identification of TMP-SMX susceptible strains. A RCT is strongly required to verify effectiveness and safety of TMP-SMX for treatment of VAP.
References
Craven DE, Hudcova J, Lei Y (2011) Diagnosis of ventilator-associated respiratory infections (VARI): microbiologic clues for trachea bronchitis (VAT) and pneumonia (VAP). Clin Chest Med 32(3):547–557. https://doi.org/10.1016/j.ccm.2011.06.001
Ramírez-Estrada S, Lagunes L, Peña-López Y, Vahedian-Azimi A, Nseir S, Arvaniti K, Bastug A, Totorika I, Oztoprak N, Bouadma L, Koulenti D, Rello J, the EU-VAE Study Investigators Group (2018) Assessing predictive accuracy for outcomes of ventilator-associated events in an international cohort: the EUVAE study. Intensive Care Med 12. https://doi.org/10.1007/s00134-018-5269-7
Wałaszek M, Różańska A, Wałaszek MZ, Wójkowska-Mach J, Polish Society of Hospital Infections Team (2018) Epidemiology of ventilator-associated pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish intensive care units in the years 2013-2015. BMC Infect Dis 18(1):308. https://doi.org/10.1186/s12879-018-3212-8
Lee MS, Walker V, Chen LF, Sexton DJ, Anderson DJ (2013) The epidemiology of ventilator-associated pneumonia in a network of community hospitals: a prospective multicenter study. Infect Control Hosp Epidemiol 34(7):657–662. https://doi.org/10.1086/670991
Li L, Dai JX, Xu L, Chen ZH, Li XY, Liu M, Wen YQ, Chen XD (2018) Antimicrobial resistance and pathogen distribution in hospitalised burn patients: a multicenter study in Southeast China. Medicine (Baltimore) 97(34):e11977. https://doi.org/10.1097/MD.0000000000011977
Bonell A, Azarrafiy R, Huong VTL, Viet TL, Phu VD, Dat VQ, Wertheim H, van Doorn HR, Lewycka S, Nadjm B (2019) A systematic review and metaanalysis of ventilator-associated pneumonia in adults in Asia; an analysis of national income level on incidence and aetiology. Clin Infect Dis 68(3):511–518. https://doi.org/10.1093/cid/ciy543
Patro S, Sarangi G, Das P, Mahapatra A, Mohapatra D, Paty BP, Chayani N (2018) Bacteriological profile of ventilator-associated pneumonia in a tertiary care hospital. Indian J PatholMicrobiol 61(3):375–379. https://doi.org/10.4103/IJPM.IJPM_487_16
Quartin AA, Scerpella EG, Puttagunta S, Kett DH (2013) A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 13:561. https://doi.org/10.1186/1471-2334-13-561
Kemnic TR, Coleman M 2018 Trimethoprim Sulfamethoxazole. StatPearls. Treasure Island (FL), StatPearls Publishing
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL (2016) Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63(5):e61–e111. https://doi.org/10.1093/cid/ciw353
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 50:3. https://doi.org/10.1183/13993003.00582-2017
Eliakim-Raz N, Hellerman M, Yahav D, Cohen J, Margalit I, Fisher S, Zusman O, Shaked H, Bishara J (2017) Trimethoprim/sulfamethoxazole versus vancomycin in the treatment of healthcare/ventilator-associated MRSA pneumonia: a case-control study. J Antimicrob Chemother 72(3):882–887. https://doi.org/10.1093/jac/dkw510
Paul M, Bishara J, Yahav D, Goldberg E, Neuberger A, Ghanem-Zoubi N, Dickstein Y, Nseir W, Dan M, Leibovici L (2015) Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 350:h2219. https://doi.org/10.1136/bmj.h2219
Hibbard ML, Kopelman TR, O’Neill PJ, Maly TJ, Matthews MR, Cox JC, Vail SJ, Quan AN, Drachman DA (2010) Empiric, broad-spectrum antibiotic therapy with an aggressive de-escalation strategy does not induce gram-negative pathogen resistance in ventilator-associated pneumonia. Surg Infect 11(5):427–432. https://doi.org/10.1089/sur.2009.046
Weiss E, Zahar JR, Lesprit P, Ruppe E, Leone M, Chastre J, Lucet JC, Paugam-Burtz C, Brun-Buisson C, Timsit JF, De-escalation Study Group (2015) Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin Microbiol Infect 21(7):649.e1–649.10. https://doi.org/10.1016/j.cmi.2015.03.013
https://sitegpr.com/fr/ (last accessed on Thursday, September 27th, 2018)
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guidelines. Ann Intern Med 158(11):825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Hedrick TL, Smith RL, McElearney ST, Evans HL, Smith PW, Pruett TL, Young JS, Sawyer RG (2008) Differences in early- and late-onset ventilator-associated pneumonia between surgical and trauma patients in a combined surgical or trauma intensive care unit. J Trauma 64(3):714–720. https://doi.org/10.1097/TA.0b013e31811ec18e
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Hoang S, Georget A, Asselineau J, Venier AG, Leroyer C, Rogues AM, Thiébaut R (2018) Risk factors for colonization and infection by Pseudomonas aeruginosa in patients hospitalised in intensive care units in France. PLoS One 13(3):e0193300. https://doi.org/10.1371/journal.pone.0193300
Crémieux AC, Muller-Serieys C, Panhard X, Delatour F, Tchimichkian M, Mentre F, Andremont A (2003) Emergence of resistance in normal human aerobic commensal flora during telithromycin and amoxicillin-clavulanic acid treatments. Antimicrob Agents Chemother 47(6):2030–2035
Merino I, Hernández-García M, Turrientes MC, Pérez-Viso B, López-Fresneña N, Diaz-Agero C, Maechler F, Fankhauser-Rodriguez C, Kola A, Schrenzel J, Harbarth S, Bonten M, Gastmeier P, Canton R, Ruiz-Garbajosa P (2018) R-GNOSIS Study Group 2018 Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother 73(11):2973–2980. https://doi.org/10.1093/jac/dky296
Montero M, Sala M, Riu M, Belvis F, Salvado M, Grau S, Horcajada JP, Alvarez-Lerma F, Terradas R, Orozco-Levi M, Castells X, Knobel H (2010) Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur J Clin Microbiol Infect Dis 29(3):335–339. https://doi.org/10.1007/s10096-009-0850-1
Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK, Canadian Critical Care Trials Group (2008) Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 23(1):18–26. https://doi.org/10.1016/j.jcrc.2008.02.001
Abbara S, Pitsch A, Jochmans S, Hodjat K, Cherrier DP, Monchi M, Vinsonneau C, Diamantis S (2019) Impact of a multimodal strategy combining a new standard of care and restriction of carbapenems, fluoroquinolones and cephalosporins, on antibiotic consumption and resistance of P. aeruginosa in an ICU. Int J Antimicrob Agents 53(4):416–422. https://doi.org/10.1016/j.ijantimicag.2018.12.001
Siber GR, Gorham CC, Ericson JF, Smith AL (1982) Pharmacokinetics of intravenous trimethoprim - sulfamethoxazole in children and adults with normal and impaired renal function. Rev Infect Dis 4(2):566–578
Young LS (1982) Trimethoprim-sulfamethoxazole in the treatment of adults with pneumonia due to Pneumocystis carinii. Rev Infect Dis 4(2):608–613
Klepser ME, Zhu Z, Nicolau DP, Banevicius MA, Belliveau PP, Ross JW, Broisman L, Quintiliani R, Nightingale CH (1996) Oral absorption of trimethoprim-sulfamethoxazole in patients with AIDS. Pharmacotherapy 16(4):656–662
Richter SS, Satola SW, Crispell EK, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Diekema DJ, Doern GV (2011) Detection of Staphylococcus aureus isolates with heterogeneous intermediate-level resistance to vancomycin in the United States. J Clin Microbiol 49(12):4203–4207. https://doi.org/10.1128/JCM.01152-11
Burnham JP, Burnham CA, Warren DK, Kollef MH (2016) Impact of time to appropriate therapy on mortality in patients with vancomycin-intermediate Staphylococcus aureus infection. Antimicrob Agents Chemother 60(9):5546–5553. https://doi.org/10.1128/AAC.00925-16
Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME, ATTAIN Study Group (2011) Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 52(1):31–40. https://doi.org/10.1093/cid/ciq031
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a distributed, controlled study. Clin Infect Dis 54(5):621–629. https://doi.org/10.1093/cid/cir895
Laohavaleeson S, Barriere SL, Nicolau DP, Kuti JL (2008) Cost-effectiveness of telavancin versus vancomycin for treatment of complicated skin and skin structure infections. Pharmacotherapy 28(12):1471–1482. https://doi.org/10.1592/phco.28.12.1471
McKinnell JA, Corman S, Patel D, Leung GH, Gordon LM, Lodise P (2018) Effective antimicrobial stewardship strategies for cost-effective utilization of telavancin for the treatment of patients with hospital-acquired bacterial pneumonia caused by Staphylococcus aureus. ClinTher 40(3):406–414.e2. https://doi.org/10.1016/j.clinthera.2018.01.010
Sharland M, Pulcini C, Harbarth S, Zeng M, Gandra S, Mathur S, Magrini N (2018 Jan) 21st WHO Expert Committee on Selection and Use of Essential Medicines: Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect Dis 18(1):18–20. https://doi.org/10.1016/S1473-3099(17)30724-7
Choquet-Kastylevsky G, Vial T, Descotes J (2002) Allergic adverse reactions to sulfonamides. Curr Allergy Asthma Rep 2(1):16–25 PMID:11895621
Rodríguez Del Águila M, González-Ramírez A (2014) Sample size calculation. Allergol Immunopathol (Madrid) 42(5):485–492. https://doi.org/10.1016/j.aller.2013.03.008
Hackshaw A (2008) Small studies: strengths and limitations. Eur Respir J 32:1141–1143. https://doi.org/10.1183/09031936.00136408
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
Because this study did not require further laboratory analysis or different clinical acts than daily clinical routine, written consent was not sought from any eligible patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strazzulla, A., Postorino, M.C., Purcarea, A. et al. Trimetoprim-sulfametoxazole in ventilator-associated pneumonia: a cohort study. Eur J Clin Microbiol Infect Dis 38, 2163–2169 (2019). https://doi.org/10.1007/s10096-019-03656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03656-2