Abstract
Tuberculosis (TB) remains one of the most deadly infections with approximately a quarter of cases not being identified and/or treated mainly due to a lack of resources. Rapid detection of TB or drug-resistant TB enables timely adequate treatment and is a cornerstone of effective TB management. We evaluated the analytical performance of a single-tube assay for multidrug-resistant TB (MDR-TB) on an experimental platform utilising RT-PCR and melting curve analysis that could potentially be operated as a point-of-care (PoC) test in resource-constrained settings with a high burden of TB. Firstly, we developed and evaluated the prototype MDR-TB assay using specimens extracted from well-characterised TB isolates with a variety of distinct rifampicin and isoniazid resistance conferring mutations and nontuberculous Mycobacteria (NTM) strains. Secondly, we validated the experimental platform using 98 clinical sputum samples from pulmonary TB patients collected in high MDR-TB settings. The sensitivity of the platform for TB detection in clinical specimens was 75% for smear-negative and 92.6% for smear-positive sputum samples. The sensitivity of detection for rifampicin and isoniazid resistance was 88.9 and 96.0% and specificity was 87.5 and 100%, respectively. Observed limitations in sensitivity and specificity could be resolved by adjusting the sample preparation methodology and melting curve recognition algorithm. Overall technology could be considered a promising PoC methodology especially in resource-constrained settings based on its combined accuracy, convenience, simplicity, speed, and cost characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) has re-emerged as the leading cause of mortality associated with an infectious disease globally causing 1.8 million deaths from an estimated 10.4 million incident of TB cases in 2015 [1]. Of these TB cases, 11% (including 0.4 million deaths) occur in HIV co-infected patients [2]. The mainstay of diagnosis in most global settings remains sputum smear microscopy which lacks both sensitivity and specificity [3, 4]. Patients, particularly children, frequently cannot freely expectorate sputum, and HIV co-infected patients produce lower TB bacterial numbers in their sputum. There remains a significant need for new rapid diagnostic systems.
More recently nucleic acid amplification (NAAT) systems such as line probe assays (LPAs) (sensitivity 86.7–100% and specificity 82.4–100%) [5] and the Xpert® MTB/RIF machine and assay (Cepheid Inc., Sunnyvale, CA, USA) (sensitivity 97.6% and specificity 99.2%) [6] have been implemented approaching microbiological culture in terms of sensitivity and specificity but offering significantly shorter turnaround times.
Innovative assays based on high-resolution melting curve analysis have been developed recently and evaluated for rapid detection of TB and rifampicin and isoniazid resistance. In these assays, fluorescent probes bind to specific gene sequences in a temperature-dependent order and measured fluorescence rises and falls as temperature increases allowing even a single-nucleotide mismatch to be detected by a characteristic fluorescence signature.
In our study, we evaluated the analytical performance of a novel assay and an experimental platform for testing multidrug-resistant tuberculosis (MDR-TB), resistant to at least rifampicin (RIF) and isoniazid (INH) that could potentially be operated as a point-of-care (PoC) test. The assay is based on a novel highly multiplexed DNA-sensing technology and melt curve analysis utilising an innovative magnetic bead extraction methodology for isolation of TB cells from sputum [7]. These have been integrated into a single-use cartridge for use within the platform which has been designed for direct processing of clinical samples without the need for user intervention.
Materials and methods
Study design
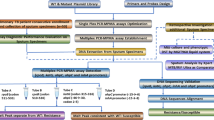
The study had two major phases. The first phase of the study was a development and evaluation of the performance of the prototype MDR-TB wet assay using the LightCycler® 2.0 instrument (Roche Diagnostics GmbH, Mannheim, Germany) comprising 120 DNA specimens (panel 1) followed by a blind validation of a prototype freeze-dried assay using a second panel (panel 2).
The second phase was a validation of the experimental platform technology on 98 clinical sputum samples comprising panel 3.
Test panels
Panel 1 included a total of 120 specimens of purified genomic DNA (gDNA) samples from Mycobacterium tuberculosis complex (Mtbc) (N = 91) and non-purified DNA from nontuberculous Mycobacteria (NTM) cultures (N = 29) (Table S1). It included duplicate samples to assess reproducibility and sample-to-sample and run-to-run variation. Within the panels, Mtbc and NTMs were identified using line probe assays (GenoType® Mycobacterium CM assay, GenoType® Mycobacterium MTBC assay, and GenoType® AS assay (Hain Lifescience GmbH, Nehren, Germany)). NTM species were additionally confirmed using Sanger sequencing of 16s RNA genes. Mutations in rpoB, katG, and the promoter region of inhA genes were characterised by whole genome sequencing (WGS).
RIF- and INH-resistant M. tuberculosis isolates within this panel (N = 80) harboured 50 distinct common and rare rpoB mutations (single, double, and triple SNPs, as well as several small non-frame shifting indels), 4 katG variants, and 4 inhA promoter polymorphisms (Tables S2 and S3).
Panel 2 contained purified gDNA samples extracted from RIF- and INH-sensitive and RIF- and INH-resistant clinical TB isolates, including 70 Mycobacterium tuberculosis isolates, 10 positive controls (M. tuberculosis H37Rv), and 10 Mtbc species (M. bovis, M. africanum, M. canetti, M. microti, M. pinnipedii), and 29 samples of nontuberculous Mycobacteria species were included in panel 1.
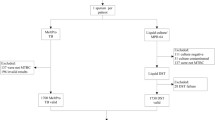
Panel 3 consisted of 98 consecutive sputum specimens collected from 98 hospitalised patients (aged ≥ 18), with pulmonary TB, at the Infectious Diseases and Tuberculosis Hospital in Vilnius, Lithuania. Residual sputum specimens (leftover from specimens collected as part of their routine clinical management) were collected consecutively between September and November 2015 until the planned number of smear positive and negative was achieved. Among 98 collected sputum specimens, 75 were smear positive and 23 were smear negative with 61 and 8 culture-positive specimens within these groups, respectively. Specimens were collected before a treatment commenced. Anonymised residual sputum specimens were tested at the local hospital using a range of molecular and phenotypic reference assays (see below).
Specimen processing for assay development and evaluation
Bacterial culture and DNA isolation (panels 1 and 2)
Isolates were cultured on Middlebrook 7H9 broth for 2 to 4 weeks (NTMs) at 30–37 °C or on Middlebrook 7H11+OACD agar plates for 4–6 weeks (Mtbc) at 37 °C. Sweeps of Mtbc colonies were harvested, resuspended in TE, and inactivated by heating at 80 °C for 50 min. After lysis by vortexing for 3 min with 0.1-mm glass beads, gDNA was purified using a DNeasy® Blood and Tissue Kit (QIAGEN GmbH, Hilden, Germany). The purified gDNA was used for PCR reactions. Alternatively, NTM colonies were resuspended in TE, mixed with an equal volume of chloroform, and heated at 80 °C for 50 min. This inactivated and lysed bacteria (non-purified DNA) was used with PCR mix.
Sputum specimens processing (panel 3)
The chemistry of sample preparation was based on a proprietary magnetic bead extraction technology (Microsens Diagnostics Ltd., London, UK): 5 ml of sputum samples was mixed with two volumes of a deactivation reagent (20% isopropanol, 2 M sodium hydroxide) and incubated for 15 min. Effective inactivation of M. tuberculosis bacteria was demonstrated using in-house viability checks before incorporating the extraction technology into the prototype platform.
Approximately 1.5 ml of decontaminated and liquefied sputum was transferred to a cartridge tube. The cartridge was inserted into the prototype instrument (run time approximately 2 h).
MDR-TB assay design
The MDR-TB assay was designed for detection of M. tuberculosis, differentiation between members of the M. tuberculosis complex (via detection of gyrB sequence differences), and detection of mutations in the rpoB, katG, and inhA genes associated with resistance to RIF and INH on primary sputum specimens. The MDR-TB assay amplifies and detects four targets in the Mtbc genome (rpoB, gyrB, katG, inhA) and Lactococcus lactis cremoris (process control for assay amplification and contamination) using four fluorophores in four optical channels (Table S4).
Development and initial evaluation of the MDR-TB assay was performed with panel 1. A validation was performed with panel 2 to determine assay analytical sensitivity and specificity using the LightCycler® 2.0 instrument (Roche Diagnostics GmbH, Nehren, Germany). PCR reactions were carried out in a final reaction volume of 20 μl (Table S5). Samples were amplified as follows: initial denaturation step at 95 °C for 3 min; 60 cycles of denaturation at 95 °C for 10 s, annealing at 68 °C for 25 s, and extension at 72 °C for 20 s followed by 95 °C 30 s; 40 °C 30 s; and ramping to 90 °C with continuous melt curve data acquisition. Total genomic DNA from H37Rv reference TB strain and nuclease-free DEPC-treated water were used as positive and negative controls, respectively. Primer and probe sequences are given in Table S6.
Freeze-dried MDR-TB PCR assay
For each reaction, 2 μl sample and 2 μl genomic Lactococcus DNA were combined with 36 μl nuclease-free DEPC-treated water and used to resuspend one freeze-dried assay cake, a lyophilised mixture containing optimised target-specific primers, buffers, salts, and enzyme. The cake was allowed to rehydrate for 1 min and mixed by pipetting. The 20 μl was transferred to a capillary and reactions loaded onto the LightCycler 2.0.
Reference tests
Phenotypic and molecular characterisation of sputum specimens (panel 3)
Reference diagnostic tests were performed on anonymised residual sputum specimens at the TB Reference Laboratory, Infectious Diseases and Tuberculosis Hospital, affiliate of Public Institution Vilnius University Hospital Santariskiu Klinikos. Specimens were processed following the standard NALC-NaOH method [8]. Concentrated sediment was resuspended in phosphate buffer and inoculated onto automated Bactec MGIT960 system tubes and LJ (Lowenstein Jensen) slants. Graded sputum smear microscopy was performed by ZN (Ziehl-Neelsen) staining [8]. Drug susceptibility testing for RIF and INH was performed on all positive cultures using the MGIT960 system (Becton Dickinson, Oxford, UK) with standardised drug concentrations [9]. DNA for GenoType assays (Hain Lifescience, Nehren, Germany) was extracted from an aliquot of resuspended sample pellet by heating for 20 min at 95 °C, followed by incubation for 15 min in an ultrasonic bath. The GenoType MTBDRplus assay was carried out as described by the manufacturer.
Ethical permission
Ethical approval for the collection of residual sputum specimens was obtained at the Infectious Diseases and Tuberculosis Hospital in Vilnius, Lithuania. Ethical approval for the whole study was received from the Imperial College London (ICL) Research Ethics Committee. Patients invited to take part in the study were provided with an information sheet and informed consent was obtained before the enrolment into the study. No patient data was recorded. All specimens were fully anonymised and personal identifiers removed so specimens could not be traced back to patients.
Data analysis
Specimen processing and data analysis for both panels 2 and 3 were performed by operators in a blinded manner with no access to reference test results.
The raw data for panel 3 generated at ICL was sent for blinded analysis using proprietary algorithms. Results (i.e. TB, NTM, isoniazid and rifampicin resistance or sensitivity) were returned to ICL and performance was compared to reference diagnostic tests. Initial data analysis and performance characteristic calculations (sensitivity, specificity, NPV, and PPV) were performed using Microsoft Excel 2010.
Results
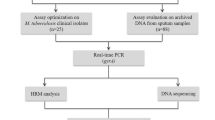
Initial evaluation of MDR-TB assay on the LightCycler 2.0 instrument
Development and initial validation of the MDR-TB assay was performed using the wet assay format on panel 1. Primers, probes, fluorophores, and their combinations were optimised to ensure optimal discrimination of all mutants from wildtype strains, identification of M. tuberculosis and M. bovis, and distinguishing them from NTMs. Probes and primers were selected and optimised, and an algorithm for identification of Mtbc species and detection of mutations in gyrB, rpoB, katG, and inhA genes using melting curve profiles (Figs. S1, S2, S3, and S4) was developed and subsequently incorporated into the MDR-TB assay analytical pipeline.
Blinded validation of MDR-TB prototype assay on the LightCycler RT-PCR system
The MDR-TB prototype assay developed on panel 1 was subsequently validated on panel 2 using the LightCycler 2.0, the testbed for the assay prototype platform. Two independent operators visually inspected melting curves and distinguished between wildtype and mutant variants of rpoB, katG, and inhA genes for Mtbc.
Sensitivity and specificity for Mtbc identification and detection of mutations of the prototype assay compared to WGS based on visual inspection of melting curves were ranging from 98.6 to 100% (Table 1). Both operators were in agreement in all but one case of Mtbc versus NTM detection; this was marked as ambiguous result and excluded from further calculations. Specificity and sensitivity for M. tuberculosis and M. bovis detection were 100%. Sensitivity for rpoB and katG mutant detection was 98.6% for both genes; the prototype assay missed the rpoB H445D mutation and one katG mutation. Specificity was 100% for all targets.
Evaluation of the experimental MDR-TB platform
Performance of the platform on panel 3 was assessed separately for smear-positive and smear-negative sputum specimens versus GenoType MTBDRplus reference test results. Interpretable results for the experimental MDR-TB assay were obtained for 87 specimens (88.8%). No results (due to an absence of internal control amplification) were recorded for 11 specimens (11.2%); these were excluded from further analysis.
The sensitivity of the prototype platform for TB detection in primary specimens was 75 and 92.6% for smear-negative and smear-positive samples respectively (Table 2). The specificity was 83.3% for smear-negative samples. The specificity for smear-positive samples could not be calculated. Among 69 smear-positive samples, one was negative by GenoType MTBDRplus and five by the MDR-TB assay but none of the negative results were negative by both tests at the same time so the specificity was non-calculable.
One of the specimens was negative (no amplification) by GenoType MTBDRplus but was identified as M. bovis by the experimental assay. The sensitivity of the LightCycler 2.0 MDR-TB assay for rifampicin and isoniazid resistance was 88.9 and 96.0%, respectively, and specificity was 87.5 and 100%, respectively (Table 2).
Analysis of discrepancies in drug susceptibility testing results between tested platform and GenoType MTBDRplus revealed the presence of both false-positive and false-negative results. Polymorphisms missed in resistant isolates included the most common mutations S315T in katG and S531L in rpoB genes (Table 3).
A separate analysis of the assay’s performance for TB and RIF and INH resistance detection conducted using MGIT culture results as a reference standard demonstrated similar sensitivity but lower specificity (Table S7 and S8).
Discussion
We assessed analytical performance of a prototype MDR-TB assay and the experimental prototype platform for rapid detection of M. tuberculosis and M. bovis and identification of resistance to key first-line drugs (RIF and INH) in clinical respiratory specimens.
Several commercial diagnostic assays for detection of Mtbc and resistance to key anti-TB drugs based on nucleic acid amplification technology (NAAT) are currently in use in diagnostic laboratories [4]. The GeneXpert test endorsed by WHO was designed to be a near point-of-care (PoC) device [9]. Validation studies acknowledged good performance characteristics of many TB assays (such as Hain Genotype MTBDRPlus/CM/AS, GeneXpert, and INNO-LiPA RifTB) but noted that further work was required to improve sensitivity on primary specimens and address other issues including heteroresistance and cross-reactivity resulting in false-positive and false-negative results for TB and drug resistance detection [10, 11].
Studies with the GeneXpert platform reported cross-reactivity with NTM strains which resulted in false-positive rifampicin resistance due to early Ct values by cross-hybridisation with NTM [12]. This indicates a need for developing an assay which is based on RT-PCR coupled with melting curve analysis, specifically high-resolution melting curve analysis (HRMA) eliminating downstream processing of PCR products (such as the hybridisation of mutants to a membrane strip used by GT Blot 20 or 48 machine (Hain Lifescience) or gel electrophoresis) and cross-contamination from amplicons by using the closed tube system [13].
The newest assays developed to address this issue such as the lab-on-chip-based platform (ST Microelectronics, Geneva, Switzerland), a molecular assay designed for detection of MDR-TB in low-income countries with an accuracy of 97.8% [14], and Xpert MTB/RIF Ultra, the upgraded version of GeneXpert recommended by WHO for children and extra-pulmonary and HIV co-infected patients utilising melt curve analysis [15]. The TB-LAMP, another closed system assay recommended as an alternative for smear microscopy in countries with an intermediate or high TB burden, has a lower sensitivity compared to GeneXpert MTB/RIF [15, 16].
The prototype platform perfectly fits into this group of rapid diagnostic tests with great potential as a PoC device based on accuracy, convenience, simplicity, and speed. Importantly, proposed platform uses the closed system meaning that reaction is performed in one single tube sealed upon transferring an original sputum into it. The whole process does not involve steps like DNA extraction, PCR amplification, and hybridisation and is not prone to amplicon contamination.
The sensitivity of the platform for smear-negative, culture-positive samples was 75% and for smear-positive, culture-positive samples was 92.6% which is slightly lower compared to GeneXpert performance reported by Boehme (76.9 and 98.3%, respectively) [17].
The sensitivity (88.9 and 96.0%) and specificity (87.5 and 100%) for RIF and INH resistance, respectively, compared to GenoType MTBDRplus assay were similar to sensitivities and specificities reported for GenoType MTBDRplus assay and slightly lower than those for INNO-LiPA RifTB and GeneXpert® MTB/RIF (for RIF only) in a recent systematic review [5]. All missed cases were associated with the most common resistant genotypes S450L and S315T in rpoB and katG genes respectively. These mutations are found in 90% of Beijing isolates and 67% of Euro-American isolates and in 74% of Beijing and 30% of Euro-American isolates, respectively [18]. However, the same wet prototype assay, performed on LightCycler 2.0 and assessed visually, gave an optimal performance with 100% specificity and sensitivity for detection of Mtbc and differentiation between Mtbc and NTMs. The high sensitivity and specificity were also achieved for detection of rpoB and katG mutations (Table 1). Thus, the suboptimal performance of the platform for INH and RIF resistance detection indicates that more work on the chemistry of sample preparation and on the melting curve reading algorithm is required. This problem was also indicated by the proportion of unreadable results (11%). It was significantly higher than the 2.4–5.9% observed in studies that reported the performance of GeneXpert [6, 17, 19].
The sample preparation protocol required 5 min hands-on work and minimum involvement and training time of a technician (nurse level). The disinfectant reagent is ready to use and the only time when the technician is potentially exposed to aerosolised organism is when the disinfectant is added to the sputum collection cup. The proposed technology does not require containment level 3 or biosafety cabinets.
Time required to analyse one to four samples (maximum) from the moment of placing the patient’s sample with a technician to receiving the result was 2 h 20 min. The hands-on time was 20 min (including 15 min incubation time) compared to 44 min of GeneXpert, 45 min of INNO-LiPA, and 50 min of GenoType MTBDRplus [5, 20].
Despite some limitations in sensitivity and specificity, which might be solved by adjusting the melting curve algorithm further, the study demonstrated that the platform might be a good alternative to already existing tests.
Authors’ conclusion
There is a number of tests available for rapid detection of drug-resistant TB. From the health economic perspective, test must be accurate and available at the lowest possible cost that permits commercial success. Thus, different test configurations may be of value to TB services and programmes in countries with different incomes, health delivery systems, and geography. For many middle-income countries, there is a health structure that better suits a more centralised diagnostic approach, whilst for others, a highly disseminated approach would be more appreciate. Different tests are needed for different environments. We do not claim that this technology is superior to the existing ones. We would rather like to see it among a great number of successful tests of different configurations helping to drive down the diagnostic costs and identify the best treatment options for TB patients.
References
World Health Organization (2015) Glocal tuberculosis report 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf. Accessed 26 May 2017
World Health Organization (2016) WHO treatment guidelines for drug- resistant tuberculosis. Accessed 26 May 2017 http://www.who.int/tb/areas-of-work/drug-resistant-tb/treatment/resources/en/
Singhal R, Myneedu VP (2015) Microscopy as a diagnostic tool in pulmonary tuberculosis. Int J Mycobacteriology. https://doi.org/10.1016/j.ijmyco.2014.12.006
Diagnostics Technology Landscape. Tuberculosis. Unitaid. Innovation in global healh (2017). Accessed 25 September 2017. https://unitaid.eu/assets/2017-Unitaid-TB-Diagnostics-Technology-Landscape.pdf
Drobniewski F, Cooke M, Jordan J, Casali N, Mugwagwa T, Broda A et al (2015) Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess 19(34):1–188
Boehme C, Nabeta P, Hillemann D et al (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363(11):1005–1015
Wang X, Zhao L, Yu X, Li Y, Ma Y, Dong L et al (2013) Bead capture increases the sensitivity of sputum microscopy for the diagnosis of tuberculosis in Beijing, China. Trans R Soc Trop Med Hyg 107(11):741–743
Tortoli E, Benedetti M, Fontanelli A, Simonetti MT (2002) Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: comparison with the radiometric BACTEC 460TB method and the agar plate method of proportion. J Clin Microbiol 40(2):607–610
Kent P T, Kubica G P (1985) Public health microbiology a guide for the level III laboratory. Division of Laboratory Training and Consultation Laboratory Program Office. Atlanta, Giorga. Accessed on 22 September 2017. https://wonder.cdc.gov/wonder/prevguid0000092/p0000092.asp#head002000000000000
Folkvardsen DB, Thomsen VO, Rigouts L, Rasmussen EM, Bang D, Bernaerts G et al (2013) Rifampin heteroresistance in Mycobacterium tuberculosis cultures as detected by phenotypic and genotypic drug susceptibility test methods. J Clin Microbiol 2013 51(12):4220–4222
Nikolayevskyy V, Balabanova Y, Simak T, Malomanova N, Fedorin I, Drobniewski F (2009) Performance of the Genotype® MTBDRPlus resistance pattern Samara, Russian Federation. BMC Clin Pathol 9:2
Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K et al (2010) Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 2010 48(1):229–237
Yin X, Zheng L, Liu Q, Lin L, Hu X, Hu Y et al (2013) High-resolution melting curve analysis for rapid detection of rifampin resistance in mycobacterium tuberculosis: a meta-analysis. J Clin Microbiol 2013 51(10):3294–3299
Cabibbe AM, Miotto P, Moure R, Alcaide F, Feuerriegel S, Pozzi G et al (2015) A lab-on-chip based platform for fast molecular diagnosis of multi-drug resistant tuberculosis. J Clin Microbiol 53(12):3876–3880
WHO Meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Accessed on 23 November 2017. http://www.who.int/tb/publications/2017/XpertUltra/en/
WHO Guidance. End TB (2017) The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis. Policy guidance. Accessed on 23 November 2017. https://www.ncbi.nlm.nih.gov/books/NBK384520/
Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R et al (2011) Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet Elsevier 377(9776):1495–1505
Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I et al (2014) Evolution and transmission of drug resistant tuberculosis in a Russian population. Nat Genet 46(3):279–286
Scott LE, McCarthy K, Gous N, Nduna M, van Rie A, Sanne I et al (2011) Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med 2011 8(7):1–11
WHO (2014) Xpert MTB/RIF implementation manual Technical and operational ‘how-to’: practical considerations. Accessed on 23 November 2017. http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf
Acknowledgements
We thank Edita Vasiliauskiene from the TB Reference Laboratory, Infectious Diseases and Tuberculosis Hospital, Vilnius University Hospital Santariskiu Klinikos, for sharing clinical sputum samples used in the assay validation. We also would like to thank Dr. Rohit Mistry for the project management at initial stage of the project.
Funding
The work was funded by a joint Innovate UK grant: Grant Number 101552; Title: Single tube Point-Of-Care multidrug-resistant tuberculosis test.
Author information
Authors and Affiliations
Contributions
NC, VN, and FD designed the study; AB performed the laboratory work and validation of experiments. NC, VN, FD, and AB interpreted the data and results and wrote the paper. HK designed, developed, and verified the MDR-TB assay; HK and RB worked on the assay optimisation and integration on the platform; GB optimised sample preparation; and WH oversaw overall assay’s development.
Corresponding author
Ethics declarations
Ethical approval for the collection of residual sputum specimens was obtained at the Infectious Diseases and Tuberculosis Hospital in Vilnius, Lithuania. Ethical approval for the whole study was received from the Imperial College London (ICL) Research Ethics Committee. Patients invited to take part in the study were provided with an information sheet and informed consent was obtained before the enrolment into the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Broda, A., Nikolayevskyy, V., Casali, N. et al. Experimental platform utilising melting curve technology for detection of mutations in Mycobacterium tuberculosis isolates. Eur J Clin Microbiol Infect Dis 37, 1273–1279 (2018). https://doi.org/10.1007/s10096-018-3246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3246-2