Abstract
John Cunningham virus (JCV) causes rare, but potentially life-threatening progressive multifocal leukoencephalopathy (PML) in natalizumab-treated multiple sclerosis (MS) patients. Beside JCV index, there is currently no other factor for further risk stratification. Because smoking was reported as potential risk factor for several viral and bacterial infections, we aimed to investigate whether smoking could increase the risk for JCV infection in MS patients. We screened our database of the MS Clinic of the Department of Neurology, Medical University of Innsbruck, Austria, for patients with known smoking status and test result for anti-JCV antibody index as determined by two-step ELISA at Unilabs, Copenhagen, Denmark. In a representative cohort of 200 MS patients with a rate of 36% current smokers plus 6% former smokers, we were not able to detect any association between smoking and JCV status. Furthermore, there was no association between smoking status and anti-JCV antibody index. Smoking does not seem to be a risk factor for JCV infection in MS patients and, therefore, does not represent a suitable marker for PML-risk stratification under treatment with natalizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a rare, but potentially life-threatening adverse event during treatment with natalizumab (Tysabri®, Biogen Idec) in MS patients [1]. It is caused by reactivation of John Cunningham virus (JCV). Measuring of anti-JCV antibodies in patients’ serum has become an important biomarker for PML risk stratification and, thus, for treatment decision making as well as for safety management during natalizumab therapy. Recent studies showed JCV prevalence rates around 55–60% in adult MS patients [2,3,4,5], of whom only a few eventually develop PML. This underscores the urgent need to further stratify anti-JCV antibody positive patients, i.e., to detect possible risk factors for JCV infection and reactivation. So far, the only confirmed marker which allows narrowing the high PML-risk group within JCV positive patients is JCV index [6].
Up to date, there is no current knowledge about possible risk factors for JCV infection. The route of transmission of JCV has not been discovered so far, whereby environmental risk factors have been discussed as well as gastrointestinal and respiratory route [7,8,9,10,11]. There is some evidence that tobacco smoking may be a risk factor for different viral infections [12], although there is no study investigating possible coincidence of JCV infection and smoking so far. Since smoking is an easily assessable risk factor, we aimed to investigate a possible coincidence of smoking and JCV infection in MS patients in order to detect a potential influence of respiratory tract in transmission of JCV.
Methods
We obtained our patient data retrospectively from the specific database used in the MS Clinic of the Department of Neurology at the Medical University of Innsbruck, Austria. All demographic and clinical data including smoking habits as well as diagnostic and treatment data have been entered in this database for more than 10 years. We included patients of whom smoking status, gathered during routine visits, was available for different time points prior, during, and after onset of MS, and of whom at least one JCV test was available. Additionally, all JCV stratify-test results including JCV index where collected in order to allow a follow-up of JCV status during treatment.
A general vote of ethics committee for use of anonymized retrospective data was obtained. Anti-JCV antibodies (IgG subclass) were routinely tested by a two-step ELISA (STRATIFY JCV DxSelect™ [13]) at Unilabs, Copenhagen, Denmark. We obtained qualitative (JCV negative/positive) as well as quantitative results, the latter expressed by an OD (optical density) which allows quantifying presence of anti-JCV antibodies and stratifying patients into negative, low positive ( <1.5) and high positive (> 1.5) for anti-JCV antibodies.
For statistical analysis, Graph Pad Prism 6 (Graphpad Software Inc., La Jolla, CA, USA) was used. Distribution of data was tested using D’Agostino-Pearson normality test. According to distribution and category, data are shown as median and range or mean ± standard deviation as appropriate. Smoking habits and JCV status were correlated using chi-square-test, group comparison of JCV index between smokers and non-smokers was performed by Mann-Whitney-test. P values of < 0.05 were considered statistically significant.
Results
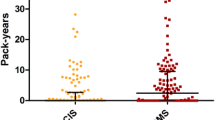
We included those patients in our analyses of whom smoking status before, during, and after onset of MS as well as at least one JCV test were available which resulted in a study cohort of n = 200. The cohort included 153 (76.5%) female and 47 (23.5%) male patients. Median age at time of first JCV testing was 35 (14–54) years. Sixty-four (32%) patients were persistently JCV negative, and 114 (57%) were persistently positive. Six patients (3%) switched from negative to positive status during follow-up testing and 16 (8%) patients converted/reconverted at least once between negative and positive around the cut-off index. Seventy-two patients (36%) were smokers, 12 (6%) stopped smoking before onset of MS and, consecutively, before JCV testing, while 116 (58%) patients where ever non-smokers. Table 1 shows results of association of smoking habits and JCV status (negative/positive), whereby there was no difference of JCV prevalence between smokers and non-smokers. Switchers of JCV status were analyzed separately. Among the six patients converting from negative to positive JCV status, five were smokers, whereas in the group of patients fluctuating around the cut-point between negative and positive, six patients smoked and ten were non-smokers. No difference of anti-JCV antibody index was found between smokers and non-smokers (Fig. 1). Median JCV index was 0.436 (0.070–4.120) for non-smokers and 0.398 (0.080–4.030) for smokers with a p value of 0.298.
When including patients who stopped smoking before JCV testing, median JCV index for ever-smokers was 0.332 (0.080–4.030) with no significant difference as compared to non-smokers (p value of 0.474).
Discussion
We investigated a representative cohort of MS patients for possible coincidence of smoking and JCV infection. Our study cohort was predominantly female according to higher prevalence of MS in women [14]. JCV prevalence at initial testing was 57% which is in agreement with previously published data [2,3,4,5]. Thirty-six percent of patients were current smokers, percentage of ever-smokers was 42%. These data coincide approximately with official smoking statistics [15]. A slightly higher prevalence of smokers in MS patients in comparison with normal population is to be assumed due to higher coincidence of psychiatric morbidity in patients with chronic diseases such as MS, depression, anxiety, and addictive behavior [16, 17]. In our study, we were not able to confirm any association of JCV infection and smoking habits, neither with JCV status (negative/positive) nor with JCV index. Transmission of JCV is still poorly understood. Since smoking with all known influences to the respiratory tract seems not to play a role for JCV infection, the theory of viral transmission via the respiratory route cannot be further supported by our study. There have been several attempts to investigate transmission route of JCV. Berger et al. [18] investigated content of JCV-DNA in body fluids such as oropharyngeal fluid, blood, and urine; however, they only detected a considerable amount of copies by polymerase chain reaction (PCR) in urine why they suggested that urine could contribute to transmission of JCV. Other authors suggest a role of respiratory transmission as well due to the presence of virus particles in stromal cells of tonsils and oropharynx [10]. Vanchiere et al. [11] investigated stool specimens, where they were able to detect presence of JCV in the gastrointestinal tract of some subjects (more children than adults) and discussed a potential role of fecal-oral transmission of polyomaviruses. By excretion in urine or stool, polyomaviruses could be released to the environment. Studies detected JCV in about 98% of sewage specimens and confirmed a high stability of the virus outside the human body [7,8,9]. Due to the ubiquity of JCV and based on sequence analyses of the virus, these authors concluded a potential role of JCV intake via contaminated water or food. Furthermore, there is evidence that JCV infection in children occurs both as vertical transmission from parents to children as well as outside family, indicated by detection of virus DNA-strain-subtypes in urine of children which were partially (in 50%) identical in parents and their children, but diverse in the other half of investigated subjects [19, 20].
Similarly, there is still poor evidence for the role of smoking in triggering or facilitating viral infections. Smoking may have different mechanisms of interference with the immune system and may affect different immunological pathways. A recent review described the current knowledge about interaction of tobacco smoking with different cell types of the innate and adaptive immune system including up- and downregulation of cytokine cascades [21]. Moreover, a decreased level of circulating immunoglobulins and depression of antibody response as well as decreased release of proinflammatory cytokines in smokers has been described [12]. Furthermore, irritation of epithelial structures of the respiratory tract by smoke may lead to adaptive responses of the immune system [22]. Altogether, nicotine and other tobacco components seem to have immunosuppressive effects which may lead to higher susceptibility to bacterial and viral infections. Several studies estimated a higher risk of upper and lower respiratory tract infection in smokers [23, 24]. For influenza, similarly, a higher incidence, and, especially more severe symptoms were found in smokers compared to non-smokers [25, 26]. Also, for HPV (human papilloma virus) and HIV (human immunodeficiency virus), a higher prevalence in smokers was described; however, particularly for these viral infections high-risk behaviors such as sexual practices have to be considered as potential confounders [12]. One study could not detect a relationship between infection with BK-virus, a polyomavirus such as JCV, and smoking as well as other demographic variables [27]. One study investigated a potential oncogenetic role of JCV in lung cancer [28]. To our knowledge, this is the only study investigating the association of JCV and smoking so far, demonstrating a lack thereof (so far there was only one study where this association was investigated as a secondary endpoint of a multivariate analysis). Since we also did not find any relation between smoking and JCV infection in a sufficiently representative cohort, we conclude that smoking habits seem not to contribute additional stratification potential for selection of a high-risk PML group in natalizumab-treated MS patients.
Furthermore, JCV infection seems to occur largely independently from modifiable risk factors.
References
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366:1870–1880. https://doi.org/10.1056/NEJMoa1107829
Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B (2014) Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol 21:299–304. https://doi.org/10.1111/ene.12304
Olsson T, Achiron A, Alfredsson L, Berger T, Brassat D, Chan A, Comi G, Eraksoy M, Hegen H, Hillert J, Jensen PEH, Moiola L, Myhr KM, Oturai A, Schippling S, Siva A, Sorensen PS, Trampe AK, Weber T, Potts J, Plavina T, Paes D, Subramanyam M, Wiendl H, Dib H, Üren D, Hemmer B, Buck D (2013) Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler 19:1533–1538. https://doi.org/10.1177/1352458513477925
Outteryck O, Ongagna JC, Duhamel A, Zéphir H, Collongues N, Lacour A, Fleury MC, Berteloot AS, Blanc F, Giroux M, Vermersch P, de Sèze J (2012) Anti-JCV antibody prevalence in a French cohort of MS patients under natalizumab therapy. J Neurol 259:2293–2298
Warnke C, Dehmel T, Posevitz-Fejfár A, Chan A, Berthele A, Schmidt S, Haas J, Kronsbein HC, Seitz F, Tackenberg B, Mäurer M, Gerbershagen K, Limmroth V, Adams O, Hartung HP, Gold R, Hemmer B, Wiendl H, Kieseier BC (2012) Anti-JC virus antibody prevalence in a German MS cohort. Mult Scler 18:1054–1055. https://doi.org/10.1177/1352458511429955
Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, Schlain B, Campagnolo D, Belachew S, Ticho B (2014) Anti–JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 76:802–812. https://doi.org/10.1002/ana.24286
Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P, Calafell F, Girones R (2001) Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol 75(21):10,290–10,299
Bofill-Mas S, Girones R (2001) Excretion and transmission of JCV in human populations. J Neurovirol 7:345–349
Bofill-Mas S, Girones R (2003) Role of the environment in the transmission of JC virus. J Neurovirol 9(Suppl 1):54–58
Jiang M, Abend JR, Johnson SF, Imperiale MJ (2009) The role of polyomaviruses inhuman disease. Virology 384:266–273. https://doi.org/10.1016/j.virol.2008.09.027
Vanchiere JA, Abudayyeh S, Copeland CM, Lu LB, Graham DY, Butel JS (2009) Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol 47:2388–2391. https://doi.org/10.1128/JCM.02472-08
Arcavi L, Benowitz NL (2004) Cigarette smoking and infection. Arch Intern Med 164:2206–2216
Lee P, Plavina T, Castro A, Berman M, Jaiswal D, Rivas S, Schlain B, Subramanyam M (2013) A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol 57:141–146. https://doi.org/10.1016/j.jcv.2013.02.002
Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, Vécsei L, Milanov I (2006) The epidemiology of multiple sclerosis in Europe. Eur J Neurol 13:700–722
Attitudes of Europeans towards tobacco 2015. Key findings of the 2015 Eurobarometer. Eurostat, May 2015.
Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, Cutter G, Reider N (2015) The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler 21:305–317. https://doi.org/10.1177/1352458514564487
Feinstein A (2011) Multiple sclerosis and depression. Mult Scler 17:1276–1281. https://doi.org/10.1177/1352458511417835
Berger JR, Miller CS, Mootoor Y, Avdiushko SA, Kryscio RJ, Zhu H (2006) JC virus detection in bodily fluids: clues to transmission. Clin Infect Dis 43:e9–12
Kitamura T, Kunitake T, Guo J, Tominaga T, Kawabe K, Yogo Y (1994) Transmission of the human polyomavirus JC virus occurs both within the family and outside the family. J Clin Microbiol 32:2359–2363
Kunitake T, Kitamura T, Guo J, Taguchi F, Kawabe K, Yogo Y (1995) Parent-to-child transmission is relatively common in the spread of the human polyomavirus JC virus. J Clin Microbiol 33:1448–1451
Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z (2017) Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget 8:268–284. https://doi.org/10.18632/oncotarget.13613
Hedström AK, Ryner M, Fink K, Fogdell-Hahn A, Alfredsson L, Olsson T, Hillert J (2014) Smoking and risk of treatment-induced neutralizing antibodies to interferon β-1a. Mult Scler 20:445–450. https://doi.org/10.1177/1352458513498635
Blake GH, Abell TD, Stanley WG (1988) Cigarette smoking and upper respiratory infection among recruits in basic combat training. Ann Intern Med 109:198–202
Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP (1993) Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health 83:1277–1283
Kark JD, Lebiush M, Rannon L (1982) Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med 307:1042–1046
Kark JD, Lebiush M (1981) Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health 71:530–532
Polz D, Morshed K, Stec A, Podsiadło Ł, Polz-Dacewicz M (2015) Do polyomavirus hominis strains BK and JC play a role in oral squamous cell carcinoma? Ann Agric Environ Med 22:106–109. https://doi.org/10.5604/12321966
Zheng H, Abdel Aziz HO, Nakanishi Y, Masuda S, Saito H, Tsuneyama K, Takano Y (2007) Oncogenic role of JC virus in lung cancer. J Pathol 212:306–315
Acknowledgements
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not report any conflicts of interest regarding this study. Outside the study, the authors report following conflicts of interest.
M. Auer has participated in meetings sponsored by and received speaker honoraria or travel funding from Novartis, Biogen, and Merck Serono.
G. Bsteh has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck Serono, Novartis, Genzyme, and Teva Ratiopharm, and received honoraria for acting as consultant for TevaPharmaceuticals Europe.
H. Hegen has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer Schering, Biogen, Merck Serono, and Novartis, and received honoraria for acting as consultant for Teva Pharmaceuticals Europe.
F. Di Pauli has received speaking honoraria or travel funding from Biogen-Idec,Roche Austria, and Sanofi-Aventis Austria.
S. Wurth has participated in meetings sponsored by, received honoraria or travel funding from Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva Ratiopharm, Allergan, Ipsen Pharma, and Roche.
T. Berger has participated in the last 2 years in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, TG Therapeutics, and TEVA.
His institution has received financial support in the last 2 years by unrestricted research grants (Biogen, Novartis, Sanofi Aventis/Genzyme, Roche, TEVA) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Roche, and Sanofi Aventis/Genzyme, TEVA.
F. Deisenhammer has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer Healthcare, Biogen Idec, Genzyme-Sanofi, Merck, Novartis Pharma, and Teva-Ratiopharm. His institution has received financial support for participation in randomized controlled trials of INFb-1b (Betaferon, Bayer Schering Pharma), INFb-1a (Avonex, Biogen Idec; Rebif, Merck Serono), glatiramer acetate (Copaxone, Teva Pharmaceuticals), and Natalizumab (Tysabri, Biogen Idec) in multiple sclerosis. He is section editor of the MSARD Journal (Multiple Sclerosis and Related Disorders).
Ethical approval and informed consent
A vote of ethics committee for use of anonymized retrospective data was obtained. All patients included in this study signed an informed consent form.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Auer, M., Bsteh, G., Hegen, H. et al. Smoking is not associated with higher prevalence of JC virus in MS patients. Eur J Clin Microbiol Infect Dis 37, 907–910 (2018). https://doi.org/10.1007/s10096-018-3204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3204-z