Abstract
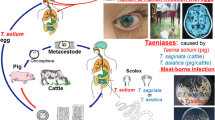
Human cysticercosis (CC) is a parasitic zoonosis caused by the larval stage (cyst) of the Taenia solium. Cysts can establish in the human central nervous system (neurocysticercosis, NCC) and other organs and tissues; they also develop in pigs, the natural intermediate host. Human taeniosis may be caused by T. solium, Taenia saginata and Taenia asiatica tapeworms; these infections are usually asymptomatic, but show a significant relevance as they perpetuate the parasites’ life cycle, and, in the case of T. solium, they are the origin of (N)CC. In European Union (EU) member states and associated countries, the occurrence of autochthonous T. solium cases is debated, and imported cases have significantly increased lately; the status of T. asiatica has been never reported, whereas T. saginata is prevalent and causes an economic impact due to condemned carcasses. Based on their effects on the EU society, the specific diagnosis of these pathologies is relevant for their prevention and control. The aims of this study were to know the diagnostic tests used in European laboratories for human taeniosis/cysticercosis by means of a questionnaire, to determine potential gaps in their detection, and to obtain preliminary data on the number of diagnosed taeniosis/CC cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human cysticercosis (CC) is a zoonotic parasitic infection caused by the larval stage (metacestode, cysticercus) of the pork tapeworm Taenia solium, formerly named Cysticercus cellulosae. These cysticerci establish in the human central nervous system (neurocysticercosis, NCC), eye, muscle, and, in rare cases, other tissues, and are a major cause of epilepsy in endemic low-income countries [1]. NCC is considered to be the most common helminth infection of the human nervous system [1, 2]. Humans acquire CC by ingesting T. solium eggs, released by themselves (auto- or self-infection) or by another tapeworm carrier [2], through fecal-oral contamination [3,4,5,6]. Humans are the unique T. solium definitive host (taeniosis), acquiring the infection by eating raw or undercooked pork harboring cysticerci; pigs are the natural intermediate host developing cysticerci by ingesting parasite eggs (porcine cysticercosis) on human feces. The maintenance of the life cycle requires a close association between humans and pigs [7]. Human taeniosis may also be caused by Taenia saginata and Taenia asiatica [8], of which the cysticerci only establish in cattle and pigs, respectively. So far, CC caused by T. saginata and T. asiatica has never been reported in humans.
Human CC occurs globally and continues to cause serious health problems [9]. The highest rates of T. solium CC are found in areas of Latin America, Asia, and sub-Saharan Africa with poor sanitation and free-ranging pigs that have access to human feces [10, 11]. In the European Union member states and associated countries (henceforth EU), T. solium was endemic in the past, although recent publications suggest that autochthonous cases may still be possible in some regions [12,13,14]. In recent years, imported CC cases have increased in parallel to the increased migration and travel [15]. Human taeniosis is not associated with major clinical symptoms, but has significant implications as it perpetuates the parasites’ life cycle, and, in the case of T. solium, causes a risk of NCC in the tapeworm carriers and people in their environment. T. solium infection is consistently classified as the most relevant food-borne parasite worldwide [16, 17]. T. saginata causes economic loss in the bovine meat sector due to condemned carcasses [18, 19].
Based on its rather rare occurrence in the EU, NCC is a challenge for care providers. NCC clinical manifestations are pleomorphic, varied and nonspecific, being related to individual differences in the number, size, location, stage of the parasite(s) and in the severity of the host’s immune response to the parasite. Although no pathognomonic clinical picture exists, in endemic regions new onset epileptic seizures and progressive crescendo headache are highly suggestive of NCC. In non-endemic regions, the diagnosis of NCC is primarily based on neuroimaging, and confirmed/aided by serology [20, 21], whereas the detection of taeniosis is most commonly made by stool microscopic examination (Taenia genus specific). Nevertheless, the early and species-specific identification of the taeniid and subsequent adapted management is crucial to avoid not only human-to-human transmission, but also human-to-pig/cattle transmission. New diagnostic tools [22], more specific and sensitive (immunological and molecular assays), have recently been developed for taeniosis and cysticercosis, however they are not yet commercially available/widely used.
Therefore, the knowledge of the in vitro diagnostic tools used in the EU for the detection of taeniosis/cysticercosis and their performances, as well as the identification/mapping of EU laboratories carrying out specific diagnosis of the disease, is of particular importance for the control, management and surveillance of these parasitic diseases.
The overall aim of the present study was to find out more about the diagnostic tests used in EU laboratories for human taeniosis/cysticercosis by means of a questionnaire in order to: (i) identify the assays offered for their examination, (ii) determine potential gaps in the techniques used by comparison with recently developed tools, and (iii) have some preliminary data on the number of taeniosis and CC cases diagnosed in the laboratories of different EU countries. In the present work, with the term taeniosis, we refer only to infections caused by cestodes of the genus Taenia.
Materials and methods
Participants
CYSTINET, the European Network on Taeniosis/Cysticercosis, consists of 27 EU countries, two EU Associated countries (Norway and Switzerland), one country (Serbia) that initiated the Stabilization and Association Process, one country (the former Yugoslav Republic of Macedonia [FYROM]) that is a candidate for accession to EU, six international partner countries and the World Health Organization (WHO) as specific organization (http://www.cost.eu), corresponds to the COST Action TD1302 (http://www.cystinet.org/). All CYSTINET members were invited via e-mail and orally at two CYSTINET meetings to fill in or forward the questionnaire link to microbiology laboratories within their specific countries.
Data collection
A set of multiple-choice questions was composed by the CYSTINET members to collect the information for the present study. Apart from some general information regarding laboratory and contact details, all questions referred to the current activity of the laboratory in the field of T. solium and T. saginata cysticercosis/taeniosis diagnostics. The questionnaire was pre-tested by CYSTINET laboratory members and thereafter finalized (Supporting information, S1 File). Since the questionnaire was composed by multiple-choice questions, the laboratory had to select the answer and if one was non-applicable, the subsequent questions related to the former remained closed. The internet-based questionnaire software SoSci Survey [23] was used to gather the data. Every laboratory could access the questionnaire with a link on the website www.soscisurvey.de. Data was downloaded from the server and processed using SPSS (SPSS Inc., released 2009, PASW Statistics for Windows, version 18.0, Chicago).
There were no restrictions for laboratories to access the questionnaire. All questionnaires were examined, including those that were not completely filled in and those from laboratories that did not agree to display their contact details. For duplicate entries, the duplicate with the least information was discarded. All answers were included anonymously in the study. Table 1 summarizes the main questions answered by the participant laboratories.
Based on the obtained information, an interactive map with the different institutions carrying out the diagnosis of the diseases in each country that agreed to display their information was made available through the COST action website (http://projects.cbra.be/cystinet/).
Results
From August 2014 to February 2016, 160 laboratories filled in the questionnaire, but only 139 laboratories agreed to have their input published (Table 1). The respondents were from 16 European countries, with Spain being the country from which most responses (n = 117) were received (Fig. 1). Most of the laboratories were microbiology laboratories. Few of them were research laboratories, which also work as reference laboratories and/or public health institutions in their countries; therefore, they also have to support other laboratories for specific problems, such as taeniosis/cysticercosis diagnosis, Trichinella outbreaks, cystic echinococcosis diagnosis, etc.
T. solium and T. saginata taeniosis diagnosis
Stool and proglottids were declared to be the samples mainly tested for the taeniosis diagnosis (Table 2A). Seventy-six laboratories (48%) stated that they handled T. solium and T. saginata taeniosis suspected samples in the same way and used the same tests, whereas eight (5%) laboratories declared they tested T. saginata and T. solium suspected samples, differently. Seventy-four laboratories (46%) did not provide any answer to this question.
Table 2B shows a summary of the tests used by the laboratories. Eighty-seven (54%) laboratories declared to test for taeniosis, whether caused by T. solium or T. saginata, but the method used was not specified. However, 78 (49%) laboratories stated the use of microscopy to diagnose taeniosis. The search for T. solium eggs in fecal samples by a microscope after stool concentration was carried out by 17 (22%) laboratories, one of which further used Ziehl-Neelsen staining [24]. Twenty-nine (37%) laboratories used stool concentration by formalin-ether or formalin-acetate as well as microscopic examination of the proglottids after ink staining. Forty-one (53%) laboratories reported to perform a microscopic examination of fresh fecal samples and a morphological identification of the proglottids; moreover, one laboratory used Carmine staining [25] and another declared to perform an ELISA as well. Two laboratories declared to use the Kato-Katz method; moreover, one of them reported to rely on histology and the other one on the cellophane-tape test [25]. For T. saginata taeniosis, five (50%) laboratories declared to perform stool concentration and two laboratories proglottid identification after ink staining [25].
Immunodiagnostic methods were declared to be used only for T. solium taeniosis in 14 (9%) laboratories, from which 11 declared to use ELISA on serum samples or whole blood, two western blot (WB) on serum samples and one ELISA plus WB on serum and cerebral spinal fluid (CSF). Seventy-one (44%) laboratories did not use any immunodiagnostic method to diagnose T. solium taeniosis and 75 (47%) did not provided any information.
Molecular methods were applied to both stool and proglottids by 15 laboratories. Nine (82%) laboratories employed conventional PCR (c-PCR) alone, c-PCR or real time PCR (RT-PCR) (one laboratory), and c-PCR and sequencing (two laboratories) for the T. solium taeniosis diagnosis. For T. saginata taeniosis, four laboratories declared using c-PCR.
Other Taenia spp. diagnosis
Twenty-six out of 160 (16%) laboratories reported the availability of diagnostic tests for other Taenia spp. Fifteen out of 26 (58%) declared microscopy as the diagnostic tool employed. Two laboratories reported staining of the proglottids, and seven used PCR for all Taenia spp.
T. solium (neuro)cysticercosis diagnosis
Serum and CSF were the preferred samples for T. solium (neuro)cysticercosis diagnosis (Table 2A), although tissue samples were also employed.
For T. solium (neuro)cysticercosis (Table 2B), 32 (20%) laboratories declared using immunodiagnostic methods based on antibody detection, and of those only three reported the use of antigen detection methods as well. Nine (28%) out of 32 laboratories used “in-house” ELISAs, from which six laboratories used crude extracts from whole cysticerci, three used cyst fluid, and one recombinant antigens as coating antigens. Commercial ELISA were from 11 companies and WB from five companies, kits were used by 28 and 14 laboratories, respectively. Indirect immuno-fluorescence assay (IFA) was employed by two laboratories, from which one declared using an “in house” IFA. An “in-house” antigen detection method for NCC, based on the monoclonal antibody, was used by one laboratory, and two laboratories stated using a commercial kit, which was specified only by one laboratory (Ag/ELISA ApDia).
Twelve (8%) laboratories declared using molecular methods for NCC; of them, nine (6%) used c-PCR and three (2%) RT-PCR in house tests. Molecular methods were used either on CSF (by two laboratories) or on cysts fluid (by one laboratory); the other nine laboratories did not specify the matrix used.
In addition, two (1.2%) out of 160 laboratories declared performing diagnosis on both bovine and porcine samples and 11 laboratories did not answer.
Performance of taeniosis/cysticercosis tests and quality assurance
Fifteen (9%) laboratories underlined relevant cross reactions in NCC antibody tests used, but the names of the kits were not reported, whereas 15 (9%) did not observe cross reactions. Cross reactions were observed with E. granulosus infection (11 laboratories), Entamoeba histolytica (one laboratory), Trichinella spp. (one laboratory), T. saginata (one laboratory) and other non-specified helminths (three laboratories).
No cross reaction was observed in antigen detection methods for T. solium NCC. Forty (25%) laboratories reported no cross reactions in T. solium taeniosis tests, eight (5%) declared cross reactions with E. granulosus (two laboratories), other non-specified helminths (two laboratories) and Entamoeba spp. (one laboratory), or did not specify the cross reactive antigens. Eight laboratories reported no cross reaction in T. saginata tests, but all of them correctly underlined being unable to microscopically distinguish taeniid species by egg morphology.
Thirty (19%) laboratories evaluated the specificity and sensitivity of the tests relying on manufacturers’ information, 17 (11%) did an “in-house” evaluation and 10 (6%) performed different procedures, i.e. external quality control (five laboratories), inter-laboratory exchange (one laboratory), and literature-based evaluation (one laboratory). Fifty-six (35%) laboratories reported that they did not perform any control, and 17 (11%) laboratories ticked “unknown” with regard to tests sensitivity and specificity.
Concerning collaborative studies, 33 (21%) laboratories declared the existence of ring trials in their countries aimed at ascertaining the quality of the T. solium and T. saginata tests, although they did not confirm their participation. Eight (24%) laboratories reported the ring trials to be organized by a National Reference Laboratory and 16 by scientific societies. Ninety-four (59%) laboratories stated lack of awareness of the organization of ring trials in their countries and 34 did not answer. Forty-four (28%) laboratories declared not being interested in participating in a European ring trial for T. solium and T. saginata tests; whereas, 92 (58%) laboratories were interested in such a collaborative study, either for T. solium and T. saginata tests (80 laboratories) or for T. solium (12 laboratories) tests alone. Twenty-five (16%) laboratories did not provide any answer to this question.
Forty-eight (30%) laboratories reported refering all T. solium/T. saginata taeniosis or T. solium NCC suspicious samples to other laboratories. Sixty (37.5%) laboratories stated referring only some samples to other laboratories. Forty-five (28%) laboratories declared to never refer samples to other laboratories. Seven (4.3%) laboratories did not answer. Sixty-nine (43.1%) laboratories referred T. solium NCC suspicious samples to a reference laboratory and 19 (11.9%) to private laboratories. Seventy-five (46.9%) and 12 (7.5%) laboratories referred T. solium/T. saginata taeniosis suspicious samples to a reference laboratory, or to private laboratories, respectively.
The reported reference laboratories were: (i) the Institute of Tropical Medicine, Antwerp, in Belgium, (ii) the Statens Serum Institut, København, in Denmark, (iii) the Department of Infectious Diseases and Tropical Medicine, University Hospital Ludwig-Maximilians-Universität, Munich, and Bernhard-Nocht Institute for Tropical Medicine, Hamburg, in Germany, (iv) the Istituto Superiore di Sanità, Rome, in Italy, (v) the Mikrobiologisk Avdeling Haukeland Universitetssykehus, Bergen, in Norway, (vi) the Instituto de Salud Carlos III, Majadahonda; Hospital Miguel Servet, Zaragoza; Hospital Universitario Virgen de la Arrixaca, Murcia; Hospital Son Espases, Mallorca; Laboratorio de Referencia de Catalunya, El Prat de Llobregat; Hospital La Fe, and Hospital Clínico, Valencia; Hospital Virgen de la Victoria, Málaga; Hospital de Basurto, Bilbao, in Spain, (vii) the Clinical Hospital of Infectious Diseases, Cluj-Napoca, in Romania, and (viii) the Hospital for Tropical Diseases, London, in the United Kingdom.
Positive samples of taeniosis and cysticercosis diagnosed during a one-year period
Twenty-four (15%) and 26 (16%) laboratories, tested NCC and taeniosis samples in the previous year, respectively (Table 3). Moreover, 54 (34%) laboratories declared to have had T. saginata taeniosis samples in the same time interval. Ninety (56%), 95 (59%) and 61 (38%) laboratories did not receive any sample for NCC, T. solium taeniosis, and T. saginata taeniosis, respectively, in the course of the previous year. The number of positive samples reported is shown in Table 3. Fifteen (9%), 13 (8%) and 9 (6%) laboratories declared being unaware of the number of NCC, T. solium taeniosis, and T. saginata taeniosis positive samples received.
Discussion
The diagnosis of T. solium (neuro)cysticercosis/T. solium taeniosis in immigrants and travelers from endemic regions, and sporadic autochthonous cases, continue to be a problem in the EU [12,13,14]. Moreover, T. saginata taeniosis persists, despite systematic meat inspection (64/433/EEC directive) [18], and the potential introduction of T. asiatica by immigrants and travelers from south-East Asia [26, 27] complicates the scenario. Since in vitro diagnostic tools are available for taeniosis and cysticercosis (Tables 4 and 5), the question arises whether laboratories from the EU are prepared to accurately diagnose human NCC and taeniid infections. Therefore, a questionnaire (Table 1) was prepared and distributed among EU laboratories to determine the present status of diagnostic tools used for the analysis of NCC, T. solium and T. saginata taeniosis.
The laboratories which filled in the questionnaire were randomly distributed in the EU (Fig. 1); indeed, most responders were from Spain (117 laboratories, 73%). Only one laboratory from Austria, Slovenia, Serbia, Switzerland and The Netherlands filled in the questionnaire. The high number of responding laboratories from Spain could be explained by an increasing interest on these pathologies due to the increase in imported cases in this country. Overall, the variation in responses could be due to (i) the different structure of health systems in the countries, more or less centralized according to the regions; (ii) lack of interest, due to the low number of cases of taeniosis/(neuro)cysticercosis diagnosed in some countries; (iii) problems with the adequacy of institutional servers, i.e. the questionnaire rejected by some of them; and (iv) no answer because of no proper distribution of the questionnaire.
As expected, stool and proglottids are the predominant samples tested for taeniosis, whereas serum is mainly tested for T. solium NCC. Several laboratories reported to employ tissue and CSF for NCC and few for T. solium taeniosis, whereas one laboratory stated testing biopsies and serum samples to diagnose T. saginata taeniosis (Table 2A). It must be stressed that biopsies, CSF and tissue samples are not adequate for taeniosis diagnosis [25]. These inadequate answers suggest that the question was not properly formulated and could have misled the participants, though the questionnaire was pre-tested; or it could be explained by a misunderstanding of these parasitic infections, with overlapping of the taeniid pathologies, or just by the lack of expertise in the diagnosis of intestinal parasites.
In general, EU laboratories seem better prepared for taeniosis diagnosis by microscopy than for (N)CC diagnosis by immunoassays [66]. This difference, observed also in the present study (Table 2B), could be explained by the low cost of basic coprological parasitological diagnostics that are routinely performed for the diagnosis of intestinal helminths, whereas “commercial” immunodetection test kits for (N)CC are expensive, have a limited shelf life, and are mainly available in laboratories, which receive a high number of requests in this specific diagnostic field.
Serological assays for T. solium, T. saginata and T. asiatica taeniosis diagnoses using recombinant antigens and immunoblots have been described [28, 67], but these assays are only used in research institutes and have not been commercialized yet.
Taeniosis
Forty-eight percent of the respondent laboratories stated handling and processing T. solium and T. saginata taeniosis suspected samples in the same way with the same techniques, whereas 5% declared processing T. saginata and T. solium suspected samples differently. These data might indicate unawareness of the risk for the analysts in processing fecal samples potentially containing T. solium eggs. Containment Level 2 facilities, equipment, and operational practices are needed [68, 69].
With respect to the methodology employed for taeniosis diagnosis, as indicated above, microscopic methods were the option most frequently chosen as opposed to immunodiagnostic or DNA methods that were used in few laboratories only (Table 4). Copro-antigen detection was used mainly in research laboratories as this technique is not commercially available. Although copro-antigen detection is considered more sensitive than microscopy, its specificity is still a matter of debate [39, 70].
It is important to stress the relevance of molecular techniques for taeniid species identification [32, 34, 35]. In the case of feces containing eggs or proglottids, genomic amplification is the preferred diagnostic option for a differential identification and the best way to recognize T. solium carriers [39]. However, only few (7, 4.3%) laboratories reported to have molecular tools to distinguish the three Taenia spp. infecting humans [40, 71]. Identification at the species level is crucial because T. solium tapeworm carriers pose an immediate threat to themselves, their household members and close contacts. The diagnosis will determine the tapeworm-carrier management that should include treatment, parasite collection, and testing for NCC of both carrier and contacts. In the case of T. saginata tapeworm carriers, their management should include treatment, and safe disposal of the tapeworm collected to avoid environmental contamination leading to bovine infections. Considering the relevance of the species-specific identification, multiplexed amplification protocols [37] would be advisable, to avoid false negative results.
With regard to microscopy, only 21% of laboratories reported their participation in ring-trials, organized by National Reference Laboratories and Scientific Societies, to follow-up quality standards (http://www.instand-ev.de/en/news.html (INSTAND), Sociedad Española de Infección y Microbiología Clínica (SEIMC), Sociedad Valenciana de Microbiología Clínica (SVAMC), Norwegian Nasjonalt Folkehelseinstituttet (FHI)). These figures are low and they suggest the need for well-organized collaborative studies to evaluate the performance of taeniosis tests used by EU laboratories.
Neurocysticercosis
Immunodiagnostic methods were employed by most of the laboratories rather than microscopy on biopsy samples or DNA detection methods for NCC (Table 5). Many laboratories used more than one test, frequently commercial kits, and few employed “in-house” assays [43, 72]. Regarding the specificity of these techniques, some laboratories highlighted relevant cross-reactions, with both protozoa and certain helminth species [73, 74], indicating no proper evaluation of the immunological tests used. Therefore, serological-ring trials with well-characterized clinical samples would be needed to determine the performances of the immunodiagnostic kits, to harmonize and standardize their use, and finally to know more about the real clinical significance of the immunodiagnostic tests employed for NCC [10, 54, 75]. Within immunodiagnostic methods, antigen-capture assays to diagnose NCC are a specific tool, mainly used on CSF. Among others, these assays allow identification and follow-up of complicated NCC infections [57, 76]. So far, the two options available were developed by European groups, the HP10 and the B158/B60 monoclonal antibody systems [77, 78], and are used for routine diagnosis and in epidemiological studies in endemic regions [60, 79].
Molecular techniques show a relevant sensitivity and excellent specificity in both taeniosis and NCC diagnosis, using different sample matrices such as CSF or tissue [40, 71]. However, the limited number of cases, lack of commercial kits and working infrastructure limit their use today.
In general, the number of positive NCC and T. solium/T. saginata taeniosis samples in the previous calendar year declared by the participating laboratories was low (Table 3). One laboratory from Spain reported the highest number of NCC and T. solium taeniosis cases (ranges from 51 to 100 and from 11 to 50, respectively). These data could indicate why the number of respondent laboratories was higher in Spain than in other European countries, because in this country the high number of Latin American immigrants could lead to an increased awareness and interest on the risk of imported T. solium human cases.
The implementation of routine ring trials, as some laboratories have already done, could be necessary to improve the standard quality level. In the case of NCC, a limited number (32, 20%) of laboratories use commercial kits and only few (9, 28%) of them use “in-house” tests that would require validation by well-organized collaborative studies.
Overall, there seem to be only a few (15, 9.3%) laboratories that have appropriate tools (all in vitro diagnostic approaches, including microscopy, and immunological and molecular assays) to identify T. solium taeniosis/NCC, which usually are located in European regions where T. solium taeniosis/NCC used to be endemic, where there is a strong travel/immigration pressure and/or where there are close relations with endemic areas by scientific networks. The results presented here are based on the replies of the participating laboratories, which were not evenly distributed over the involved countries, and as such the high number of laboratories responding from Spain has an influence on the results. The information obtained about the taeniosis/NCC tests used in the laboratories will be a valuable contribution for microbiology units to find support when they need it.
In addition, we suggest refreshing the knowledge on T. solium taeniosis/NCC infections as the prevalence of the disease seems rather low in Europe despite the fact that there is some evidence that NCC may actually be on the rise [80]. Some laboratories highlighted that they did not see cases anymore; however, we must be alert and ready for their diagnosis and surveillance. In the case of T. saginata infections, similar initiatives (e.g., knowledge refreshing on parasite transmission, risk factors, good practices, diagnostic tools) need to be applied, as T. saginata taeniosis persists in the EU countries despite an integrated approach among all stakeholders [18].
References
Del Brutto OH (2005) Neurocysticercosis. Semin Neurol 25:243–251
Zammarchi L, Strohmeyer M, Bartalesi F, Bruno E, Muñoz J, Buonfrate D, Nicoletti A, García HH, Pozio E, Bartoloni A, COHEMI Project Study Group (2013) Epidemiology and management of cysticercosis and Taenia solium taeniasis in Europe, systematic review 1990–2011. PLoS One 8:e69537
Sarti-Gutierrez EJ, Schantz PM, Lara-Aguilera R, Gomez Dandoy H, Flisser A (1988) Taenia solium taeniasis and cysticercosis in a Mexican village. Trop Med Parasitol 39:194–198
Murrell KD (2005) Epidemiology of taeniosis and cysticercosis. In: Murrell KD (ed.) WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis,World Health Organisation for Animal Health (OIE), Paris, pp 27–43
Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, Moulton LH, Villaran MV, Montano SM, Gonzalez AE, Cysticercosis Working Group in Peru (2009) Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS Negl Trop dis 3:e371
O’Neal SE, Townes JM, Wilkins PP, Noh JC, Lee D, Rodriguez S, Garcia HH, Stauffer WM (2012) Seroprevalence of antibodies against Taenia solium cysticerci among refugees resettled in United States. Emerg Infect Dis 18:431–438
Garcia HH, Rodriguez S, Friedland JS, Cysticercosis Working Group in Peru (2014) Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunol 36:388–396
Eom KS (2006) What is Asian Taenia? Parasitol Int 55:S137–S141
Carabin H, Krecek RC, Cowan LD, Michael L, Foyaca-Sibat H, Nash T, Willingham AL (2006) Estimation of the cost of Taenia solium cysticercosis in eastern Cape Province, South Africa. Tropical Med Int Health 11:906–916
Garcia HH, Nash TE, Del Brutto OH (2014) Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13:1202–1215
Winkler AS, Richter H (2015) Landscape analysis: management of neurocysticercosis with an emphasis on low- and middle-income countries. World Health Organization, Geneva
Devleesschauwer B, Allepuz A, Dermauw V, Johansen MV, Laranjo-González M, Smit GS, Sotiraki S, Trevisan C, Wardrop NA, Dorny P, Gabriël S (2017) Taenia solium in Europe: Still endemic? Acta Trop 165:96–99
Fabiani S, Bruschi F (2013) Neurocysticercosis in Europe: still a public health concern not only for imported cases. Acta Trop 128:18–26
van de Pol LA, van Doeveren TE, van der Kuip M, Wolf NI, Vermeulen RJ (2015) Pediatric neurocysticercosis: three cases presented in the Netherlands with divergent clinical presentations. Neuropediatrics 46:130–133
Gabriël S, Johansen MV, Pozio E, Smit GS, Devleesschauwer B, Allepuz A, Papadopoulos E, van der Giessen J, Dorny P (2015) Human migration and pig/pork import in the European Union: What are the implications for Taenia solium infections? Vet Parasitol 213:38–45
WHO (2014) Multicriteria-based ranking for risk management of food-borne parasites. Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO), Rome
Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou XN, Fèvre EM, Sripa B, Gargouri N, Fürst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A, de Silva N (2015) World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS med 12:e1001920
Dorny P, Praet N (2007) Taenia saginata in Europe. Vet Parasitol 149:22–24
de Paulan SC, Gonzáles RM, Peralta LA, Vicentini-Oliveira JC, Biondi GF, Conde ES, Parkhouse RM, Nunes CM (2013) Usefulness of serological ELISA assay for Taenia saginata to detect naturally infected bovines. Rev Bras Parasitol vet 22:270–275
Winkler AS, Willingham AL 3rd, Sikasunge CS, Schmutzhard E (2009) Epilepsy and neurocysticercosis in sub-Saharan Africa. Wien Klin Wochenschr 121:3–12
Winkler AS (2012) Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health 106:261–274
World Health Organization (2016) Taenia solium Taeniasis/Cysticercosis diagnostic tools. Report of a stakeholder meeting. Geneva, 17–18 December 2015. WHO Press, Geneva, pp 26
Leiner D J (2014) SoSci Survey (Version 2.6.00-i) [Computer software]. Available at https://www.soscisurvey.de /
Jimenez JA, Rodriguez S, Moyano LM, Castillo Y, García HH, Cysticercosis Working Group in Peru (2010) Differentiating Taenia eggs found in human stools: does Ziehl-Neelsen staining help? Tropical Med Int Health 15:1077–1081
Garcia LS (2001) Diagnostic medical parasitology. ASM Press, Washington, D.C.
Robertson LJ, Sprong H, Ortega YR, van der Giessen JW, Fayer R (2014) Impacts of globalisation on foodborne parasites. Trends Parasitol 30:37–52
Galán-Puchades MT, Fuentes MV (2014) Taenia asiatica: left out by globalisation? Trends Parasitol 30:54–55
Levine MZ, Calderón JC, Wilkins PP, Lane WS, Asara JM, Hancock K, Gonzalez AE, Garcia HH, Gilman RH, Tsang VC (2004) Characterization, cloning, and expression of two diagnostic antigens for Taenia solium tapeworm infection. J Parasitol 90:631–638
Handali S, Klarman M, Gaspard AN, Dong XF, Laborde R, Noh J, Lee YM, Rodriguez S, Gonzalez AE, Garcia HH, Gilman RH, Tsang VC, Wilkins PP (2010) Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vaccine Immunol 17:631–637
Allan JC, Avila G, Garcia NJ, Flisser A, Craig PS (1990) Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101:473–477
Guezala MC, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, Allan JC, Craig PS (2009) Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg 81:433–437
Harrison LJ, Delgado J, Parkhouse RM (1990) Differential diagnosis of Taenia saginata and Taenia solium with DNA probes. Parasitology 100:459–461
Zarlenga DS, McManus DP, Fan PC, Cross JH (1991) Characterization and detection of a newly described Asian taeniid using cloned ribosomal DNA fragments and sequence amplification by the polymerase chain reaction. Exp Parasitol 72:174–183
Chapman A, Vallejo V, Mossie KG, Ortiz D, Agabian N, Flisser A (1995) Isolation and characterization of species-specific DNA probes from Taenia solium and Taenia saginata and their use in an egg detection assay. J Clin Microbiol 33:1283–1288
Gonzalez LM, Montero E, Harrison LJ, Parkhouse RM, Garate T (2000) Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol 38:737–744
González LM, Montero E, Puente S, López-Velez R, Hernández M, Sciutto E, Harrison LJ, Parkhouse RM, Gárate T (2002) PCR tools for the differential diagnosis of Taenia saginata and Taenia solium taeniasis/cysticercosis from different geographical locations. Diagn Microbiol Infect Dis 42:243–249
Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Qiu D, Mamuti W, Craig PS, Ito A (2004) DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol 42:548–553
Sato MO, Sako Y, Nakao M, Wandra T, Nakaya K, Yanagida T, Ito A (2011) A possible nuclear DNA marker to differentiate the two geographic genotypes of Taenia solium tapeworms. Parasitol Int 60:108–110
Praet N, Verweij J, Mwape K, Phiri I, Muma J, Muma JB, Zulu G, van Lieshout L, Rodriguez-Hidalgo R, Benitez-Ortiz W, Dorny P, Gabriël S (2013) Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Tropical Med Int Health 18:608–614
Roelfsema JH, Nozari N, Pinelli E, Kortbeek LM (2016) Novel PCRs for differential diagnosis of cestodes. Exp Parasitol 161:20–26
Nkouawa A, Sako Y, Li T, Chen X, Wandra T, Swastika IK, Nakao M, Yanagida T, Nakaya K, Qiu D, Ito A (2010) Evaluation of a loop-mediated isothermal amplification method using fecal specimens for differential detection of Taenia species from humans. J Clin Microbiol 48:3350–3352
Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, Verastegui M, Wilkins P, Tsang VC, Cysticercosis Working Group in Peru (2001) Short report: transient antibody response in Taenia solium infection in field conditions-a major contributor to high seroprevalence. Am J Trop Med Hyg 65:31–32
Tsang VC, Brand JA, Boyer AE (1989) An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159:50–59
Chung JY, Bahk YY, Huh S, Kang SY, Kong Y, Cho SY (1999) A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J Infect Dis 180:1307–1315
Greene RM, Hancock K, Wilkins PP, Tsang VC (2000) Taenia solium: molecular cloning and serologic evaluation of 14- and 18-kDa related, diagnostic antigens. J Parasitol 86:1001–1007
Sako Y, Nakao M, Ikejima T, Piao XZ, Nakaya K, Ito A (2000) Molecular characterization and diagnostic value of Taenia solium low-molecular-weight antigen genes. J Clin Microbiol 38:4439–4444
Hancock K, Pattabhi S, Greene RM, Yushak ML, Williams F, Khan A, Priest JW, Levine MZ, Tsang VC (2004) Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol 133:115–124
Ferrer E, González LM, Foster-Cuevas M, Cortéz MM, Dávila I, Rodríguez M, Sciutto E, Harrison LJ, Parkhouse RM, Gárate T (2005) Taenia solium: characterization of a small heat shock protein (Tsol- sHSP35.6) and its possible relevance to the diagnosis and pathogenesis of neurocysticercosis. Exp Parasitol 110:1–11
Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC (2006) Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol 147:109–117
Ferrer E, Bonay P, Foster-Cuevas M, González LM, Dávila I, Cortéz MM, Harrison LJ, Parkhouse RM, Gárate T (2007) Molecular cloning and characterisation of Ts8B1, Ts8B2 and Ts8B3, three new members of the Taenia solium metacestode 8 kDa diagnostic antigen family. Mol Biochem Parasitol 152:90–100
Salazar-Anton F, Lindh J (2011) Taenia solium: a two-dimensional western blotting method combined with the use of an EST-library for the identification of immunogenic proteins recognized by sera from neurocysticercosis patients. Exp Parasitol 128:371–376
Ferrer E, Sánchez J, Milano A, Alvarez S, La Rosa R, Lares M, González LM, Cortéz MM, Dávila I, Harrison LJ, Parkhouse RM, Gárate T (2012) Diagnostic epitope variability within Taenia solium 8 kDa antigen family: implications for cysticercosis immunodetection. Exp Parasitol 130:78–85
Corstjens PL, de Dood CJ, Priest JW, Tanke HJ, Handali S, Cysticercosis Working Group in Peru (2014) Feasibility of a lateral flow test for neurocysticercosis using novel up-converting nanomaterials and a lightweight strip analyzer. PLoS Negl Trop Dis 8:e2944
Noh J, Rodriguez S, Lee YM, Handali S, Gonzalez AE, Gilman RH, Tsang VC, Garcia HH, Wilkins PP (2014) Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol 52:1429–1434
Correa D, Sandoval MA, Harrison LJ, Parkhouse RM, Plancarte A, Meza-Lucas A, Flisser A (1989) Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg 83:814–816
Garcia HH, Gonzalez AE, Gilman RH, Bernal T, Rodriguez S, Pretell EJ, Azcurra O, Parkhouse RM, Tsang VC, Harrison LJ, Cysticercosis Working Group in Peru (2002) Circulating parasite antigen in patients with hydrocephalus secondary to neurocysticercosis. Am J Trop Med Hyg 66:427–430
Fleury A, Garcia E, Hernández M, Carrillo R, Govezensky T, Fragoso G, Sciutto E, Harrison LJ, Parkhouse RM (2013) Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS Negl Trop Dis 7:e2096
Jansen F, Dorny P, Berkvens D, Van Hul A, Van den Broeck N, Makay C, Praet N, Gabriël S (2016) Assessment of the repeatability and border-plate effects of the B158/B60 enzyme-linked-immunosorbent assay for the detection of circulating antigens (Ag-ELISA) of Taenia saginata. Vet Parasitol 227:69–72
Carabin H, Millogo A, Cissé A, Gabriël S, Sahlu I, Dorny P, Bauer C, Tarnagda Z, Cowan LD, Ganaba R (2015) Prevalence of and factors associated with human Cysticercosis in 60 villages in three provinces of Burkina Faso. PLoS Negl Trop Dis 9:e0004248
Gabriël S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS (2012) Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis 6:e1851
Alexander AM, Prabhakaran V, Rajshekhar V, Muliyil J, Dorny P (2010) Long-term clinical evaluation of asymptomatic subjects positive for circulating Taenia solium antigens. Trans R Soc Trop Med Hyg 104:809–810
Almeida CR, Ojopi EP, Nunes CM, Machado LR, Takayanagui OM, Livramento JA, Abraham R, Gattaz WF, Vaz AJ, Dias-Neto E (2006) Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatry Clin Neurosci 256:307–310
Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L, Bouteille B (2011) Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol 49:195–200
Yera H, Dupont D, Houze S, Ben M'rad M, Pilleux F, Sulahian A, Gatey C, Gay Andrieu F, Dupouy-Camet J (2011) Confirmation and follow-up of neurocysticercosis by real-time PCR in cerebrospinal fluid samples of patients living in France. J Clin Microbiol 49:4338–4340
Hernández M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, Garate T, Sciutto E (2008) Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol 102:317–323
Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142:w13727
Jeon HK, Eom KS (2009) Immunoblot patterns of Taenia asiatica taeniasis. Korean J Parasitol 47:73–77
World Health Organization (2004) Laboratory biosafety manual, 3rd edn. WHO Press, Geneva, p 186
Public Health Agency of Canada (2012) Taenia solium. Pathogen safety data sheet - Infectious substances. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/taenia-solium-eng.php
Tembo A, Craig PS (2015) Taenia saginata taeniosis: copro-antigen time-course in a voluntary self- infection. J Helminthol 89:612–619
González LM, Villalobos N, Montero E, Morales J, Sanz RA, Muro A, Harrison LJ, Parkhouse RM, Gárate T (2006) Differential molecular identification of Taeniid spp. and Sarcocystis spp. cysts isolated from infected pigs and cattle. Vet Parasitol 142:95–101
Carod JF, Randrianarison M, Razafimahefa J, Ramahefarisoa RM, Rakotondrazaka M, Debruyne M, Dautigny M, Cazal P, Andriantseheno ML, Charles ER (2012) Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis 72:85–89
Hernández-Cruz E, González-Cabriales JJ, Ordaz-Pichardo C, de la Cruz-Hernández NI, Flores-Gutiérrez GH (2009) Development of an immunobinding dot-blot assay as an alternative for the serodiagnosis of human cysticercosis. J Helminthol 83:333–337
Sloan L, Schneider S, Rosenblatt J (1995) Evaluation of enzyme-linked immunoassay for serological diagnosis of cysticercosis. J Clin Microbiol 33:3124–3128
Carpio A, Fleury A, Hauser WA (2013) Neurocysticercosis: five new things. Neurol Clin Pract 3:118–125
Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, Gonzalez AE, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru (2009) Detection of Taenia solium antigens and anti-T. Solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 199:1345–1352
Harrison LJ, Joshua GW, Wright SH, Parkhouse RM (1989) Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol 11:351–370
Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J (1989) Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol 76:269–274
Cortez Alcobedes MM, Boggio G, Guerra Mde L, de Gavidia MR, Rojas Reyes GC, Ferrer E, Lares M, Alviarez Y, Harrison LJ, Parkhouse RM (2010) Evidence that active transmission of porcine cysticercosis occurs in Venezuela. Trop Anim Health Prod 42:531–537
Del Brutto OH (2012) Neurocysticercosis in Western Europe: a re-emerging disease? Acta Neurol Belg 112:335–343
Acknowledgments
This manuscript represents a collaborative work within the framework of CYSTINET, the European network on taeniosis/cysticercosis, COST ACTION TD1302. We would like to thank the European Microbiology laboratories that kindly participated and filled in the diagnostic questionnaire, as it was the core of the present review.
Author information
Authors and Affiliations
Contributions
Conceived and designed the review: MAG, TG, JB, BD, SAS, VS, MJP, AL, EP, PD, SG, ASW. Performed the questionnaire: MAG, TG, JB, BD, SAS, VS, MJP, AL, EP, PD, SG, ASW. Analyzed the data: MAG, TG, JB, BD. Wrote the review: MAG, TG, JB, BD, SAS, VS, MJP, AL, EP, PD, SG, ASW. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Funding
The present work was finding by the European Union, Cooperation in Science and Technology (COST) Actions, COST Action TD1302 “European Network on Taeniosis/Cysticercosis”.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was not required for the present review.
Electronic supplementary material
ESM 1
(PDF 112 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gómez-Morales, M.A., Gárate, T., Blocher, J. et al. Present status of laboratory diagnosis of human taeniosis/cysticercosis in Europe. Eur J Clin Microbiol Infect Dis 36, 2029–2040 (2017). https://doi.org/10.1007/s10096-017-3029-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3029-1